Calcite phase spherical porous calcium carbonate granule and preparation method thereof
A technology of porous calcium carbonate and calcite, applied in calcium carbonate/strontium/barium, chemical instruments and methods, calcium/strontium/barium compounds, etc., can solve problems such as poor dispersion, not suitable for biomedical applications, and serious powder agglomeration , to achieve the effects of mild preparation process, low energy consumption, promotion of attachment and proliferation, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
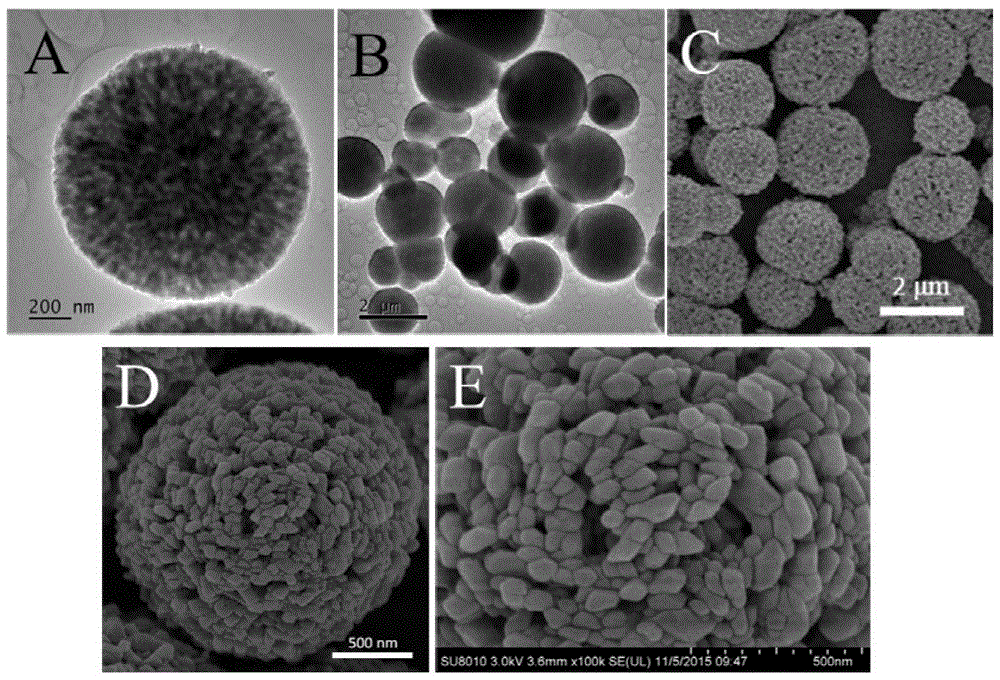
[0048] 10g CaCl 2 2H 2 O was dissolved in 400mL ethanol, and the above-mentioned mixed solution A was put into a glass jar. Put the ammonium bicarbonate powder into another 300mL glass jar. The above reaction glass containers were placed together in a sealed desiccator at 30° C. for 168 h, and a white precipitate was formed in the transparent solution. The precipitate was collected by centrifugation and dried under vacuum at 25°C to obtain a white powder. Then the obtained white powder was re-immersed in 40 mL of water / ethanol mixed solution F (containing 0.8 mL of water), left standing at 30° C. for 168 h, centrifuged, and dried to obtain calcite-phase spherical porous calcium carbonate particles. Such as figure 1 As shown in the TEM (A, B) and SEM (C, D, E) images, the material is composed of a pore structure obtained by the accumulation of rhombohedral calcium carbonate grains, spherical and round, with high crystallinity and three-dimensional penetration of the pores ...
Embodiment 2
[0050] 3.2g CaCl 2 2H 2 O was dissolved in 400mL ethanol, and the above-mentioned mixed solution A was put into a glass jar. Put the ammonium bicarbonate powder into another 300mL glass jar. The above reaction glass containers were placed together in a sealed desiccator at 30° C. for 72 hours, and a white precipitate was formed in a transparent solution. The precipitate was collected by centrifugation and dried under vacuum at 25°C to obtain a white powder. Then the obtained white powder was re-immersed in 40 mL of water / ethanol mixed solution F (containing 0.8 mL of water), left standing at 30° C. for 168 h, centrifuged and dried to obtain calcite-phase spherical porous calcium carbonate particles. The obtained material is composed of a pore structure obtained by stacking calcite-phase calcium carbonate crystal grains, is spherical and round, and has a particle diameter of less than 3 μm, and the particle diameter of the calcite-phase calcium carbonate crystal grains is 50...
Embodiment 3
[0052] 16g CaCl 2 2H 2O was dissolved in 400 mL of ethanol, and the above mixed solution A was filled into a glass jar. Put the ammonium bicarbonate powder into another 300mL glass jar. The above reaction glass containers were placed together in a sealed desiccator at 30° C. for 240 h, and a white precipitate was formed in the transparent solution. The precipitate was collected by centrifugation and dried under vacuum at 25°C to obtain a white powder. Then the obtained white powder was re-immersed in 40 mL of water / ethanol mixed solution F (containing 2 mL of water), left standing at 30° C. for 48 h, centrifuged, and dried to obtain calcite-phase spherical porous calcium carbonate particles. The obtained material is composed of a pore structure obtained by stacking calcite-phase calcium carbonate crystal grains, is spherical and round, and has a particle diameter of less than 3 μm, and the particle diameter of the calcite-phase calcium carbonate crystal grains is 50-200 nm....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com