A method of preparing battery-grade high-purity manganese sulfate by utilizing low-grade manganese ore
A low-grade manganese ore and manganese sulfate technology, applied in the direction of manganese sulfate, etc., can solve the problems of high-purity impurity removal agent consumption, insignificant removal effect, poor effect, etc., achieve remarkable impurity removal effect, good iron removal effect, easy The effect of industrial scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
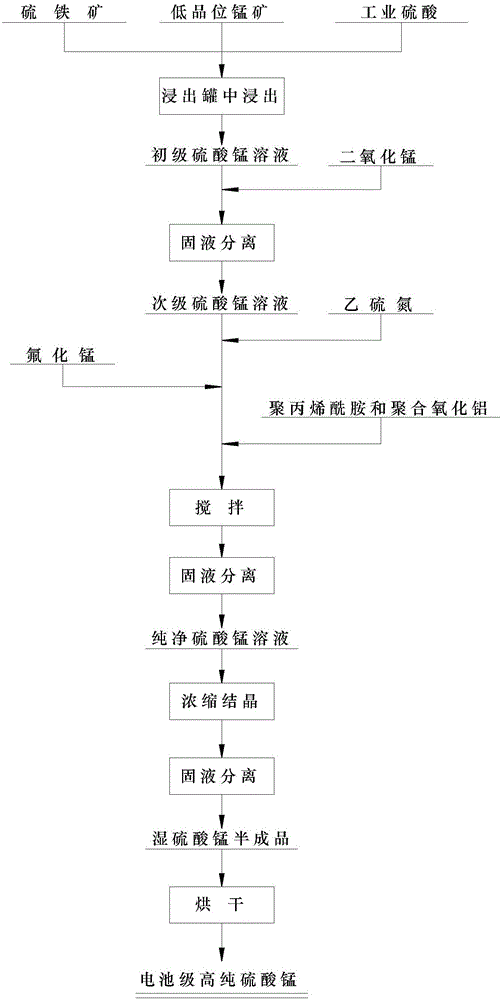
[0040]Choose low-grade manganese ore with a manganese content of 18% as the manganese ore raw material, mix low-grade manganese ore, pyrite, and industrial sulfuric acid in a mass ratio of 1:0.26:0.30, and put the prepared materials into the leaching tank After chemical leaching and solid-liquid separation, a primary manganese sulfate solution is obtained, and the concentration of the primary manganese sulfate is 250-280 g / L. Add manganese dioxide to the primary manganese sulfate solution, and adjust the pH value of the solution to 4.2, add manganese dioxide as an iron oxide remover, oxidize ferrous iron to ferric iron, regenerate ferric hydroxide to precipitate and remove iron, The iron removal effect is good; and then the solid-liquid separation is carried out to obtain the secondary manganese sulfate solution. In the secondary manganese sulfate solution, add ethyl disulfide nitrogen, according to the heavy metal content in the secondary manganese sulfate solution, the addit...
Embodiment 2
[0042] Choose low-grade manganese ore with a manganese content of 20% as the manganese ore raw material, mix low-grade manganese ore, pyrite, and industrial sulfuric acid in a mass ratio of 1:0.28:0.32, and put the prepared materials into the leaching tank After chemical leaching and solid-liquid separation, a primary manganese sulfate solution is obtained, and the concentration of the primary manganese sulfate is 250-280 g / L. Add manganese dioxide to the primary manganese sulfate solution, and adjust the pH value of the solution to 4.3, add manganese dioxide as an iron oxide remover, oxidize ferrous iron to ferric iron, regenerate ferric hydroxide to precipitate and remove iron, The iron removal effect is good; and then the solid-liquid separation is carried out to obtain the secondary manganese sulfate solution. Add ethylsulfide nitrogen in the secondary manganese sulfate solution, according to the heavy metal content in the secondary manganese sulfate solution, the addition...
Embodiment 3
[0044] Choose low-grade manganese ore with a manganese content of 25% as the manganese ore raw material, mix low-grade manganese ore, pyrite, and industrial sulfuric acid in a mass ratio of 1:0.3:0.35, and put the prepared materials into the leaching tank After chemical leaching and solid-liquid separation, a primary manganese sulfate solution is obtained, and the concentration of the primary manganese sulfate is 250-280 g / L. Adding manganese dioxide to the primary manganese sulfate solution, and adjusting the pH value of the solution to 4.5, adding manganese dioxide as an iron oxide remover, oxidizing ferrous iron to ferric iron and regenerating ferric hydroxide to precipitate and remove iron, The iron removal effect is good; and then the solid-liquid separation is carried out to obtain the secondary manganese sulfate solution. In the secondary manganese sulfate solution, add ethyl disulfide nitrogen, according to the heavy metal content in the secondary manganese sulfate sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com