Method for synthesizing 2,5-dichloroaniline by micro-channel reactor
A microchannel reactor, the technology of dichloroaniline, is applied in chemical instruments and methods, chemical/physical/physical-chemical reactors, preparation of organic compounds, etc. Sufficient, obvious magnifying effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
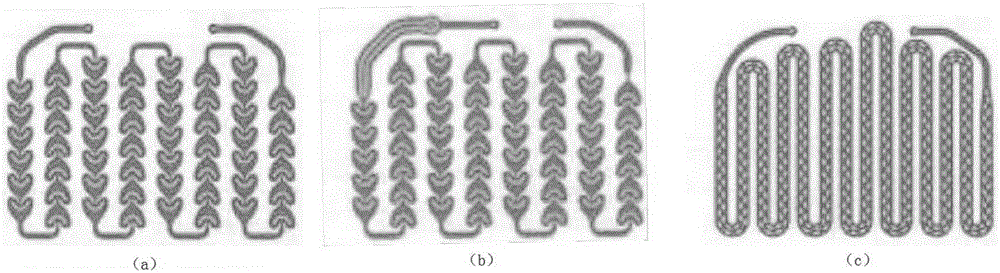
Image
Examples
Embodiment 1
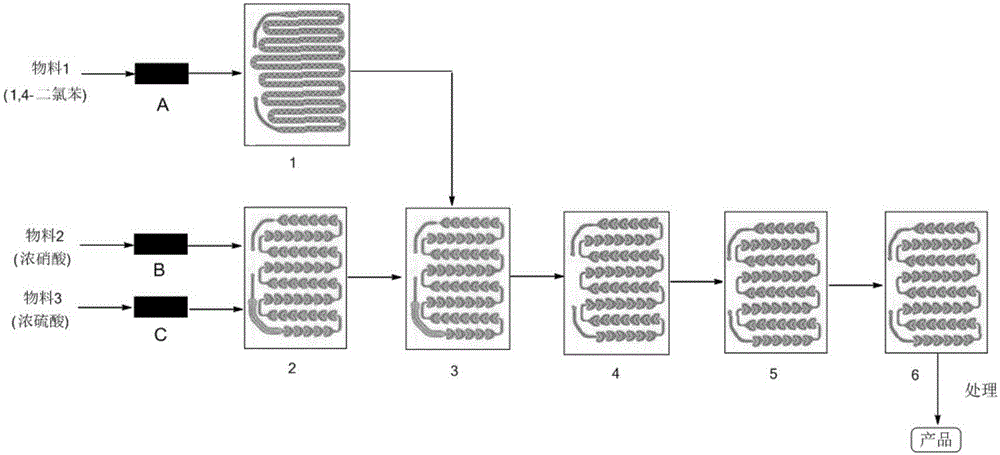
[0053] (1) Weigh 300g of 1,4-dichlorobenzene and dissolve it in 2L of dichloromethane as material 1, measure 400ml of concentrated nitric acid as material 2, and measure 600mL of concentrated sulfuric acid as material 3;
[0054] (2) The flow velocity of control material 1 is 15ml / min; The flow velocity of control material 2 is 8min / min; The flow velocity of control material 3 is 12min / min; Reaction temperature is 35 ℃, and the mol ratio of o-difluorobenzene and nitric acid is 1 :2.5; The mol ratio of nitric acid and the vitriol oil is 1:2.0; The residence time of reaction is 70s;
[0055] (3) When each material in the reactor reaches a steady state, collect the reaction liquid flowing out from the reactor outlet, and feed 120 minutes of material 1 (that is, 1800ml of material 1, 260.0g of 1,4-dichlorobenzene) to correspond to The reaction solution of the reaction solution is an example, separate liquid, keep the organic phase, extract the acid layer once with 500ml of dichlor...
Embodiment 2
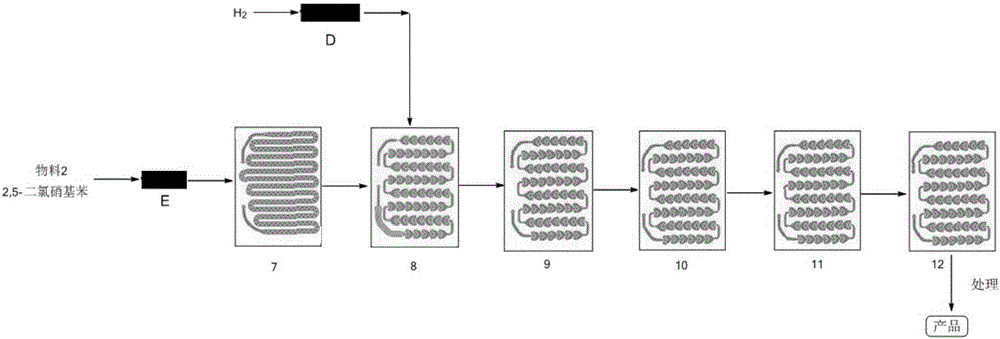
[0060] In the screening and optimization process of process parameters, the parameters such as the concentration of reaction raw materials, molar ratio, residence time have been explored, and the corresponding parameters of corresponding steps are changed on the basis of the operation steps of Example 1, and other parameter conditions are all constant. The results are summarized as follows:
[0061] 1. Nitrification reaction:
[0062]
[0063] 2. Catalytic hydrogenation reaction:
[0064]
[0065]
[0066] 3. Pd / C recovery application experiment:
[0067]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com