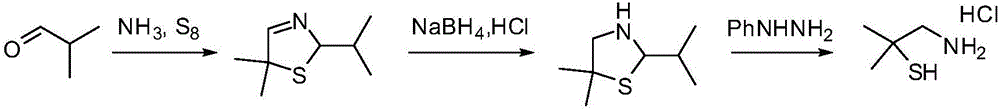
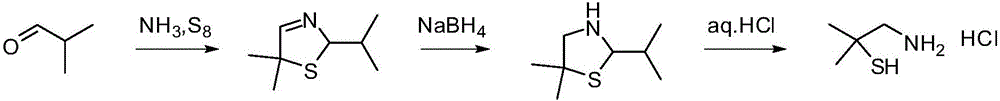
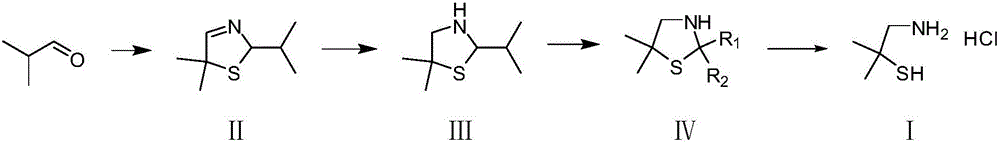
Dimethyl cysteamine hydrochloride synthetic process
A technology of dimethyl cysteamine hydrochloride and dimethyl, which is applied in the field of synthesizing dimethyl cysteamine hydrochloride to reduce operating intensity, improve reaction yield and product content, and avoid the use of pressure vessels Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 5,5-Dimethyl-2-isopropylthiazolidine (III) (79.5g), toluene (280g), phenylhydrazine (48.6g) and hydrochloric acid (700g) were added to the glass reactor in turn, heated to reflux , the reaction was incubated for 2 hours. Cool to room temperature, separate the water phase and extract it once with toluene, then add acetone (43.5g) to the water phase, adjust the pH to 10 with liquid caustic soda, stir at room temperature for 2 hours, extract twice with toluene, combine the organic phases, wash with water, remove Dissolved and distilled under reduced pressure to obtain 62.1 g of 2,2,5,5-tetramethylthiazolidine with a yield of 85.7% and a content of 97.0%.
[0049] 1H NMR (CDCl3, 400 MHz) δ: 3.02 (s, 2H), 2.63 (s, 1H), 1.59 (s, 6H), 1.44 (s, 6H).
[0050] MS: [M+1]+=145.9
[0051] The above-mentioned 2,2,5,5-tetramethylthiazolidine and concentrated hydrochloric acid (230.0g) were successively added to the there-necked flask, and the temperature was raised to 95° C. to reac...
Embodiment 2
[0055] 5,5-Dimethyl-2-isopropylthiazolidine (III) (79.5g), toluene (280g), phenylhydrazine (48.6g) and hydrochloric acid (700g) were added to the glass reactor in turn, heated to reflux , the reaction was incubated for 2 hours. The temperature was cooled to room temperature, the water phase was separated and extracted once with toluene, then methyl ethyl ketone (54.0 g) was added to the water phase, the pH was adjusted to 10 with liquid caustic soda, stirred at room temperature for 2 hours, extracted twice with toluene, combined with the organic phases and washed with water, Precipitated and distilled under reduced pressure to obtain 66.0 g of 2,5,5-trimethyl-2-ethylthiazolidine with a yield of 83.0% and a content of 97.0%.
[0056] 1HNMR(CDCl3, 400MHz)δ: 3.08-2.93(m, 2H), 2.56(s, 1H), 1.92-1.71(m, 2H), 1.53(s, 3H), 1.45(s, 3H), 1.39(s) , 3H), 1.01 (t, J=7.6Hz, 3H)
[0057] MS: [M+1]+=160.2
[0058] The above-mentioned 2,5,5-trimethyl-2-ethylthiazolidine and concentrated hy...
Embodiment 3
[0062] 5,5-Dimethyl-2-isopropylthiazolidine (III) (79.5g), toluene (280g), phenylhydrazine (48.6g) and hydrochloric acid (700g) were added to the glass reactor in turn, heated to reflux , the reaction was incubated for 2 hours. Cool to room temperature, separate the water phase and extract it once with toluene, then add cyclohexanone (73.5g) to the water phase, adjust the pH to 10 with liquid alkali, stir at room temperature for 2 hours, extract twice with toluene, combine the organic phases and wash with water , precipitation, and distillation under reduced pressure to obtain 79.5 g of 2,2-dimethyl-1-thio-4-azaspiro[4.5]decane with a yield of 86% and a content of 96.6%.
[0063] 1HNMR(CDCl3, 400MHz)δ: 2.99(s, 2H), 2.56(s, 1H), 1.90-1.86(m, 2H), 1.76-1.70(m, 4H), 1.60-1.34(m, 9H), 1.31 -1.18(m,1H)
[0064] MS: [M+1]+=186.0
[0065] The above-mentioned 2,2-dimethyl-1-thio-4-azaspiro[4.5]decane and concentrated hydrochloric acid (351.0 g) were successively added to the three-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com