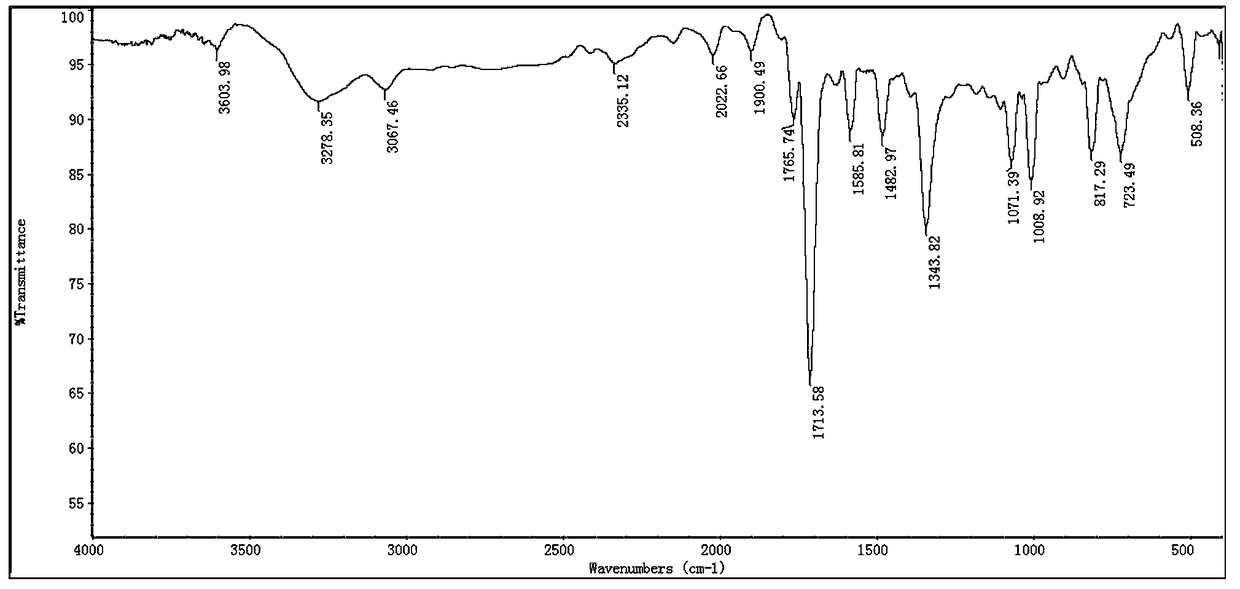
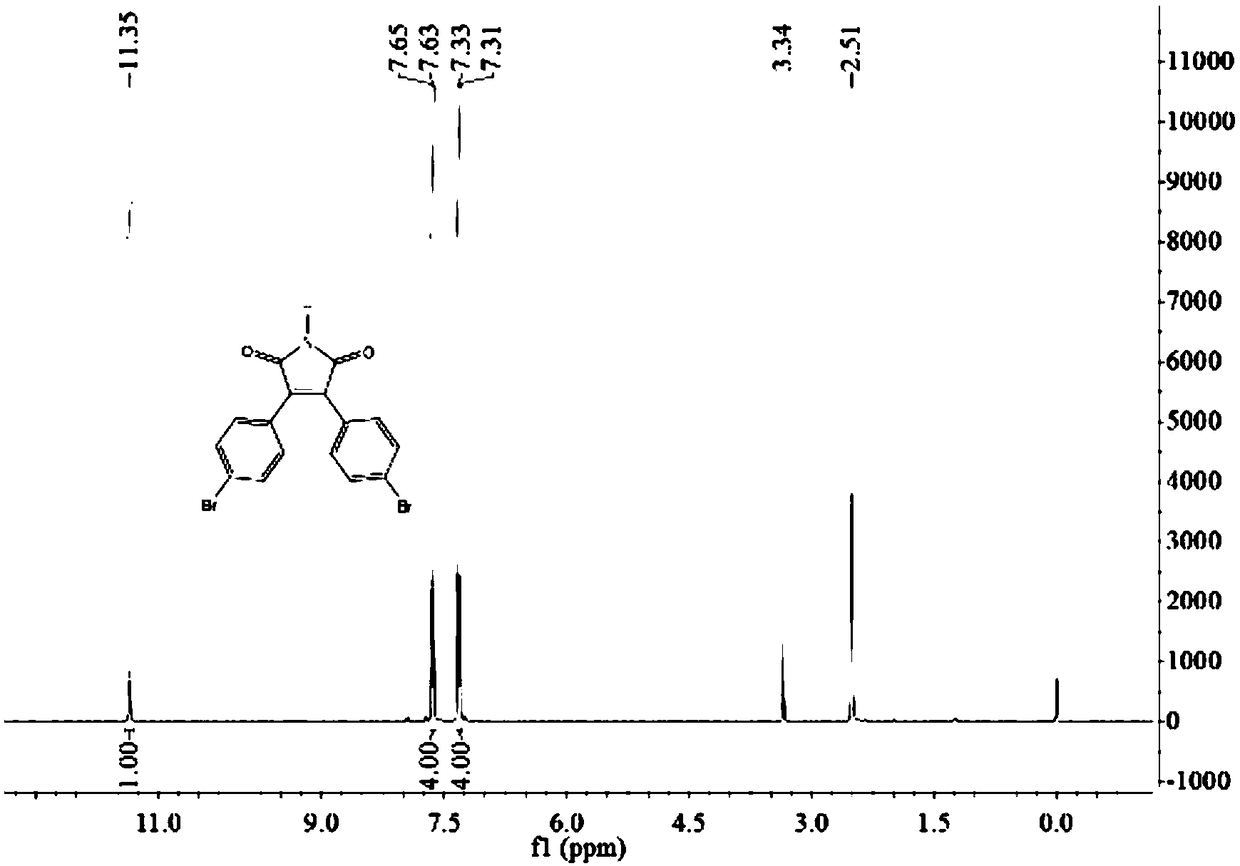
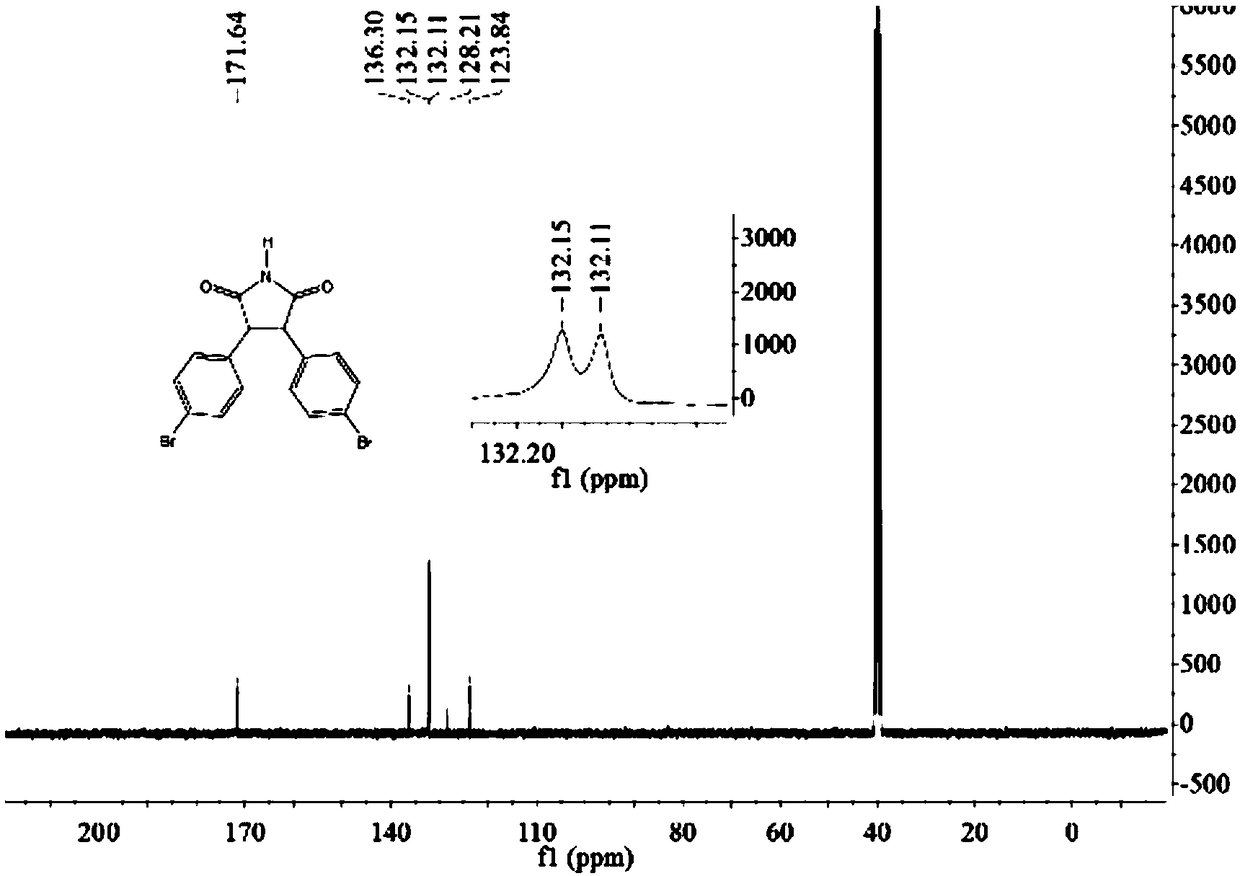
A kind of preparation method of 3,4-bis(4-bromophenyl)-1h-pyrrole-2,5-dione
A technology of bromophenyl and diketone, which is applied in the field of diphenyl-1H-pyrrole-2,5-dione and its preparation, can solve the problems of expensive catalyst palladium salt, harsh reaction conditions, and many reaction steps , to achieve the effect of cheap raw materials, short reaction route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 34.2g (0.2mol) of 4-bromoaniline, 250mL of water, and 88.0g (0.4mol) of 40% fluoroboric acid aqueous solution were added dropwise to a solution of 27.6g (0.4mol) of sodium nitrite and 100mL of water under stirring at 0°C. After the addition was completed, the mixture was raised to room temperature and continued to stir for 2 h. After the reaction was completed, it was filtered with suction to obtain 45.4 g of 4-bromophenyldiazotetrafluoroborate as a white solid with a yield of 84.0%, which was directly used in the next reaction.
Embodiment 2
[0036] 17.1g (0.1mol) of 4-bromoaniline, 150mL of water, 22.0g (0.1mol) of 40% fluoroboric acid aqueous solution were added dropwise at 5°C with a solution of 8.3g (0.12mol) of sodium nitrite and 50mL of water, dropwise After the addition was completed, the mixture was raised to room temperature and continued to stir for 2 hours. After the reaction was completed, it was filtered with suction to obtain 23.9 g of white 4-bromophenyldiazonium tetrafluoroborate with a yield of 88.5%, which was directly used in the next reaction.
Embodiment 3
[0038] Take maleimide 0.97g (0.01mol), cuprous chloride 0.099g (0.001mol), TMEDA 0.116g (0.001mol) into a 50mL round bottom flask, add DMF 15mL, and stir well. Then 2.7 g (0.01 mol) of 4-bromophenylfluoroborate diazonium salt in Example 1 was added in batches, and reacted for 6 hours. TLC monitoring, the reaction is complete, add 10 mL of water, stir for 5 minutes, extract with ethyl acetate, dry the organic phase over anhydrous sodium sulfate, and recrystallize from 95% ethanol to obtain a yellow solid 3,4-bis(4-bromophenyl)-1H- Pyrrole-2,5-dione 1.13g, yield 28%, mp: 216-218°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com