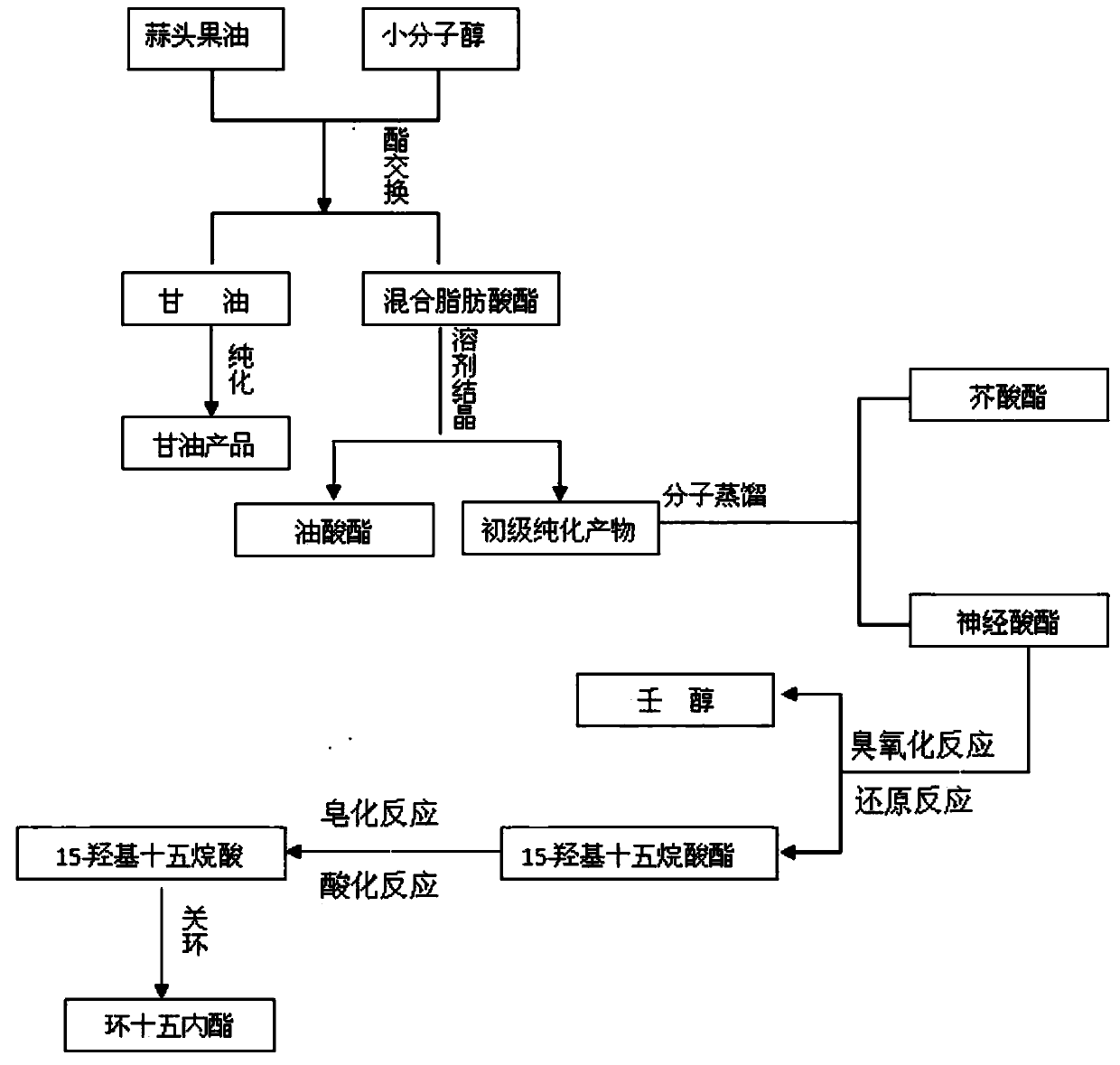
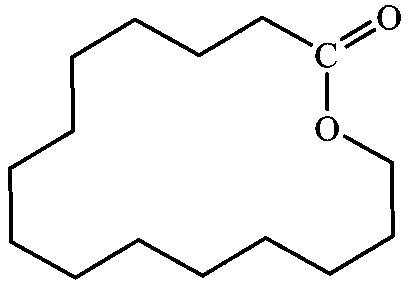
Method for preparing macrocyclic musk-cyclopentadecalactone by using garlic fruit oil
A technology of cyclopentadecanolide and garlic fruit oil, which is applied in the direction of organic chemistry, can solve the problems of decreased purity, low yield of cyclopentadecanolide, difficult separation and purification, etc., so as to solve many side reactions and improve application value , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Step (1) prepare raw material by following weight portion:
[0036] 100 parts of garlic oil, 30 parts of methanol, Na 2 O / MgO-Al 2 O 3 0.8 part, anhydrous Na 2 SO 4 6 parts, 100 parts of acetone, 130 parts of ethyl acetate, NaBH 4 10 parts, KOH 25 parts, H 3 PO 4 10 parts, solid acid SO 4 2- / Fe 2 O 3 4 parts and 50 parts of n-hexane.
[0037] Step (2) Esterification of garlic oil:
[0038] Under agitation, the garlic oil, methanol, Na 2 O / MgO-Al 2 O 3 It was sequentially added to a 250mL three-necked flask heated by a water bath at 70°C. After the addition of the materials, the reaction was continued for 5h, then the reaction product was poured into a separatory funnel, and distilled water was added to wash 3 times, 40mL each time. After the layers were separated in the separatory funnel, the water layer was released, the upper ester layer was poured out, and anhydrous Na was added. 2 SO 4 After removing the residual water, when the ester layer is cle...
Embodiment 2
[0051] Step (1) prepare raw material by following weight portion:
[0052] 100 parts of garlic oil, 40 parts of ethanol, K 2 O / MgO-Al 2 O 3 1.5 parts, anhydrous Cu 2 SO 4 10 parts, 120 parts of acetone and ethyl acetate mixed solvent with a volume ratio of 1:1, 150 parts of chloroform and petroleum ether mixed solvent with a volume ratio of 1:3, KBH 4 25 parts, 20 parts NaOH, H 2 SO 4 13 parts, 5 parts of concentrated sulfuric acid, 60 parts of petroleum ether.
[0053] Step (2) Esterification of garlic oil:
[0054] Under stirring, combine garlic oil, ethanol, K 2 O / MgO-Al 2 O 3 It was sequentially added to a 250mL three-necked flask heated by a water bath at 85°C. After the addition of the materials, the reaction was continued for 5.5 hours. Then, the reaction product was poured into a separatory funnel, and distilled water was added for washing 3 times, each time being about 40 parts by weight. After the layers were separated in the separating funnel, the water ...
Embodiment 3
[0066] Step (1) prepare raw material by following weight portion:
[0067] 100 parts of garlic oil, 50 parts of propanol, 2.5 parts of CaO / activated clay, anhydrous CaCl 2 7 parts, 170 parts mixed solvent of ethanol and ethyl acetate in a volume ratio of 1:2, 150 parts of anhydrous methanol and diethyl ether mixed solvent in a volume ratio of 1:2, KBH 4 25 parts, 20 parts of NaOH, 17 parts of p-toluenesulfonic acid, 5 parts of concentrated hydrochloric acid, and 60 parts of mixed solvent of acetone and petroleum ether in a volume ratio of 3:1.
[0068] Step (2) Esterification of garlic oil
[0069] Under stirring, add garlic oil, propanol and CaO / activated clay in turn to a 250mL three-necked flask heated in a 92°C water bath. After adding the materials, continue the reaction for 6h, and then pour the reaction product into a separatory funnel, Add distilled water to wash 3 times, about 40 parts by weight each time. After the layers were separated in the separatory funnel, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com