Selenium-containing compound and use thereof
A compound and application technology, applied in the field of selenium-containing compounds and their preparation, can solve the problems of side effects, limited anti-cancer spectrum, inability to prevent cancer cells from spreading to other organs, and achieve the effect of simple preparation method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
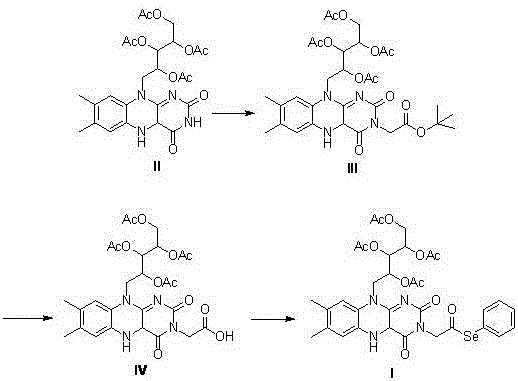
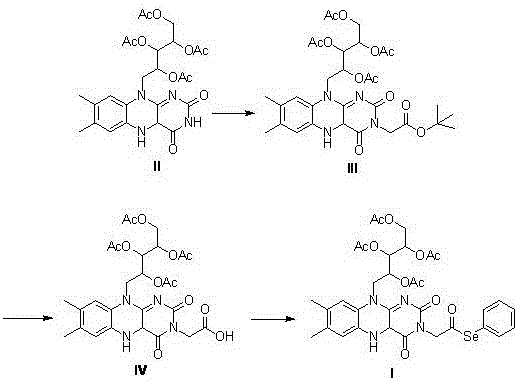
[0040] Example 1: Synthesis of 2',3',4',5'-tetraacetyl-riboflavin (Compound II)
[0041] Add riboflavin (5.0g, 13mmol), 40ml of anhydrous acetic anhydride and 10ml of pyridine in a three-necked flask, heat to 50°C, pass through argon protection, stir for 24 hours, add saturated ammonium chloride solution and ethyl acetate After separation, the aqueous phase was extracted with ethyl acetate (3×60ml), the organic phase was collected, dried over anhydrous magnesium sulfate, filtered with suction, and distilled under reduced pressure to obtain a crude product, which was subjected to column chromatography (mobile phase: ethyl acetate: Petroleum ether = 5:1 (V:V)) to obtain 6.10 g of yellow powdery solid (compound II), with a yield of 86%.
[0042] nuclear magnetic resonance 1 H NMR (400 MHz, CDCl 3 ) δ: 9.11 (br s, 1H), 7.93 (s, 1H),7.52 (s, 1H), 5.59 (br d, J = 9 Hz, 1H), 5.41- 5.39 (m, 1H), 5.34-5.31 (m,1H), 4.85 (br s, 2H), 4.36 (dd, J 1 =12Hz; J 2 =3 Hz, 1H), 4.18(dd, ...
Embodiment 2
[0044] Example 2: Synthesis of 2',3',4',5'-tetraacetyl-N(3)-tert-butoxyacetyl riboflavin (compound III)
[0045] Add 5ml of anhydrous DMF to a three-necked flask, sequentially add compound II (544 mg, 1.0 mmol), potassium carbonate (166 mg, 1.2 mmol), catalytic amount of potassium iodide, stir for 30 min under the protection of argon, and then slowly dropwise add dissolved 2 - tert-butyl bromoacetate (1 mL, 6.9 mmol) in anhydrous DMF 10 mL, heated to 40° C. and protected from light for 20 h. After the reaction is complete, extract with dichloromethane (3×20ml), collect the organic phase, wash with saturated sodium bicarbonate (10mL), water (10mL), saturated brine (10mL) successively, add anhydrous magnesium sulfate to the organic phase and dry , Suction filtration and distillation under reduced pressure to obtain crude product, column chromatography (mobile phase: ethyl acetate: dichloromethane = 1:1 (V:V)) to obtain 0.52 g of yellow powdery solid (compound III), yield 79% . ...
Embodiment 3
[0048] Example 3: Synthesis of 2',3',4',5'-tetraacetyl-N(3)-riboflavin acetic acid (Compound IV)
[0049] Add compound III (200 mg, 0.3 mmol) and 1 ml of anhydrous dichloromethane into a 100 ml three-neck flask, pass through argon to protect and cool to 0 °C, add 0.3 ml of trifluoroacetic acid, heat up to 50 °C and stir for 5 h. After the reaction is over, pour the reaction solution into ice water, adjust the pH to 5 with saturated sodium bicarbonate solution, add dichloromethane for extraction, collect the organic phase and wash it with saturated brine, then with water, and dry the collected organic phase with anhydrous magnesium sulfate , filtered with suction, dried in vacuo, and evaporated to dryness. Column chromatography (ethyl acetate:ethanol=1:1 (V:V)) gave 120 mg of a light yellow solid (compound IV), yield: 66%.
[0050] nuclear magnetic resonance 1 H NMR (400 MHz, D 2 O) δ: 7.88 (s, 1H), 7.77 (s, 1H), 5.69-5.64(m, 1H), 5.54 (t, J = 6 Hz, 1H), 5.51-4.47(m, 1H), 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com