Serum albumin with metal chelating function as well as preparation method and application in inhibition for aggregation of beta-amyloid proteins
A serum albumin and amyloid technology, applied in the field of biomedicine, can solve the problems of limited metal ion binding ability, insufficient Aβ aggregation inhibition effect, adsorption inhibition of Aβ aggregation, etc. The effect of chelating ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
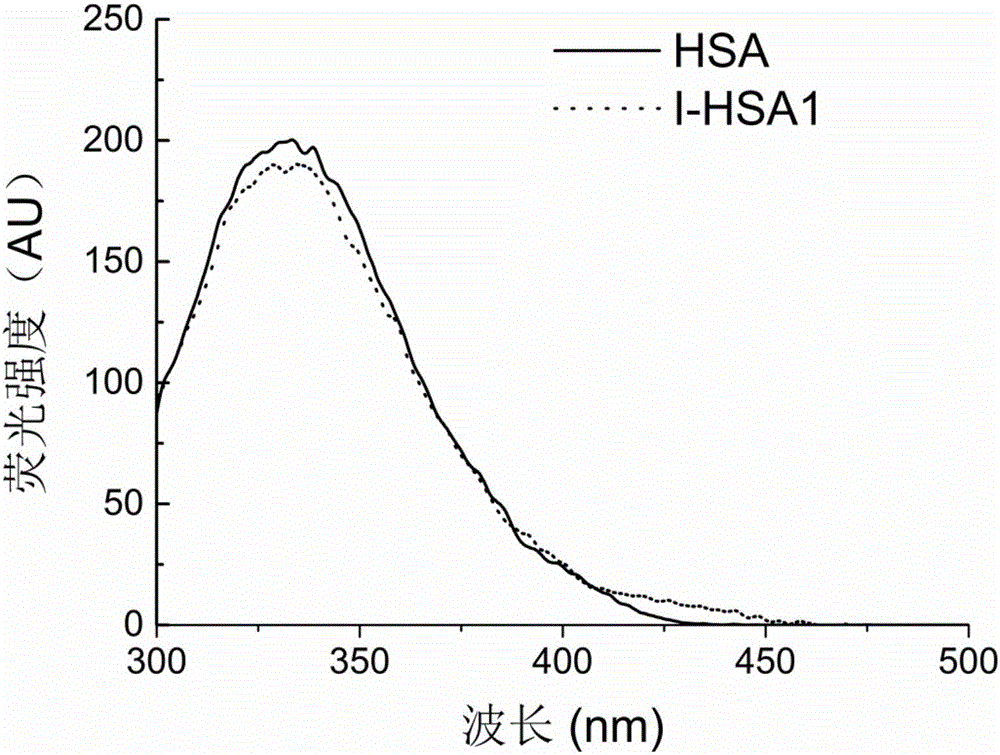
[0041] Example 1: Synthesis and characterization of metal chelating functional serum albumin with a molar ratio of iminodiacetic acid to serum albumin of 5
[0042] Weigh 20 mg of serum albumin (HSA), dissolve it in 20 mL of boric acid-borax buffer (20 mmol / L, pH 8.0) to obtain a serum albumin solution with a concentration of 1 mg / mL, and add 55 μL of 1,4-butanediol diglycidyl ether. The above mixture was stirred and reacted at 120rpm and 25°C for 8h. After the reaction, the free 1,4-butanediol diglycidyl ether in the system was removed through a SephadexG-25 gel filtration column, and the mobile phase was boric acid-borax buffer (20mmol / L, pH 8.0). Weigh 0.2 mg of iminodiacetic acid powder and add it to the solution obtained in the previous step. The above mixture was stirred and reacted at 120 rpm and 25° C. for 24 h. After the reaction, the free iminodiacetic acid in the system was removed through a SephadexG-25 gel filtration chromatographic column, and the mobile phase...
Embodiment 2
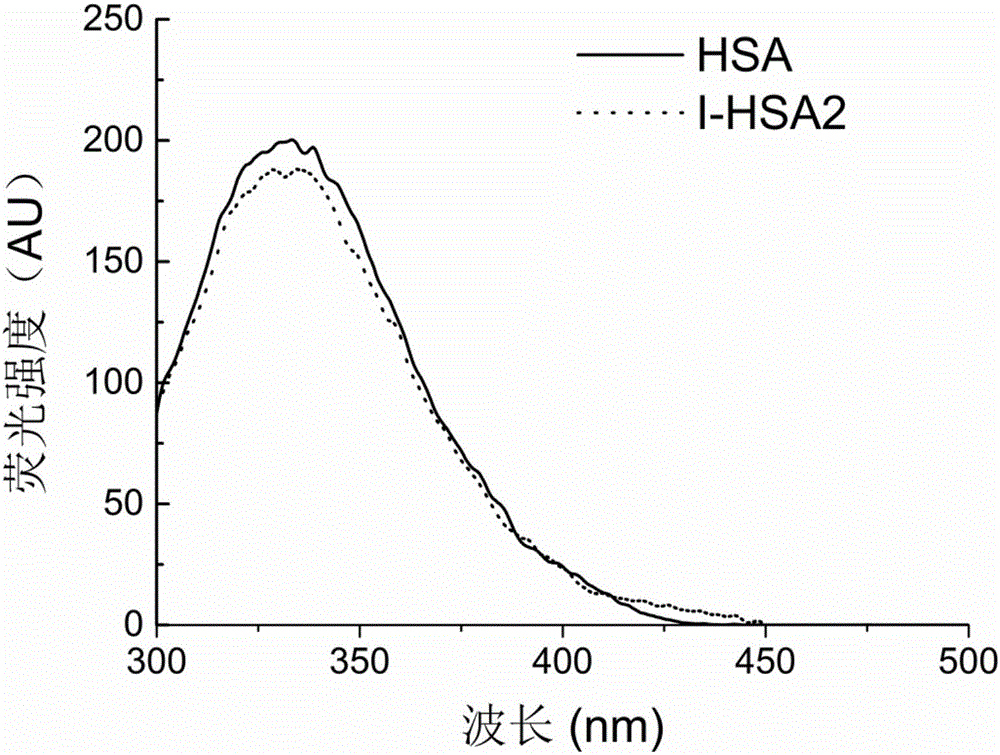
[0048] Example 2: Synthesis and characterization of metal chelating functional serum albumin with a molar ratio of iminodiacetic acid to serum albumin of 50
[0049] 200 mg of serum albumin was weighed and dissolved in 20 mL of boric acid-borax buffer (20 mmol / L, pH 8.5) to obtain a serum albumin solution with a concentration of 10 mg / mL. , and add 825 μL of 1,4-butanediol diglycidyl ether. The above mixture was stirred and reacted at 120rpm and 37°C for 10h. After the reaction, the free 1,4-butanediol diglycidyl ether in the system was removed by SephadexG-25 gel filtration chromatography column, and the mobile phase was boric acid-borax buffer (20mmol / L, pH 8.5). Weigh 19.8 mg of iminodiacetic acid powder and add it to the solution obtained in the previous step. The above mixture was stirred and reacted at 120 rpm and 37° C. for 36 h. After the reaction, remove free iminodiacetic acid in the system through a SephadexG-25 gel filtration chromatographic column, the mobile p...
Embodiment 3
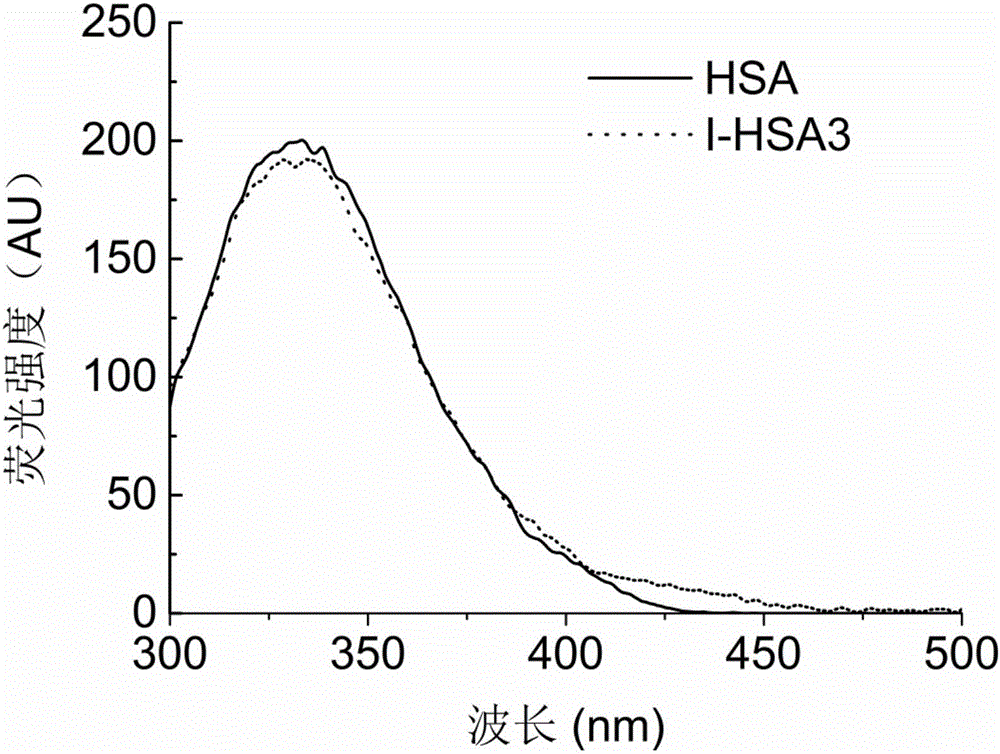
[0055] Example 3: Synthesis and characterization of acidified serum albumin with a molar ratio of iminodiacetic acid to serum albumin of 400
[0056] Weigh 400 mg of serum albumin, dissolve it in 20 mL of boric acid-borax buffer (20 mmol / L, pH 9.0) to obtain a serum albumin solution with a concentration of 20 mg / mL, and add 2200 μL of 1,4-butanediol disulfide glyceryl ether. The above mixture was stirred and reacted at 120rpm and 50°C for 12h. After the reaction, the free 1,4-butanediol diglycidyl ether in the system was removed by SephadexG-25 gel filtration chromatography column, and the mobile phase was boric acid-borax buffer solution (20mmol / L, pH 9.0). Weigh 316.8 mg of iminodiacetic acid powder and add it to the solution obtained in the previous step. The above mixture was stirred and reacted at 120rpm and 50°C for 48h. After the reaction, the free iminodiacetic acid in the system was removed by SephadexG-25 gel filtration chromatographic column, the mobile phase was w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com