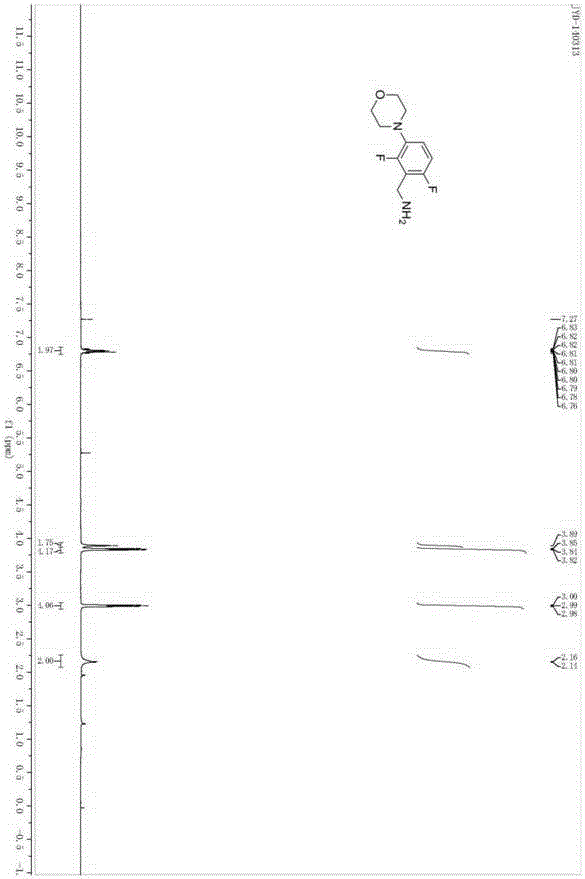
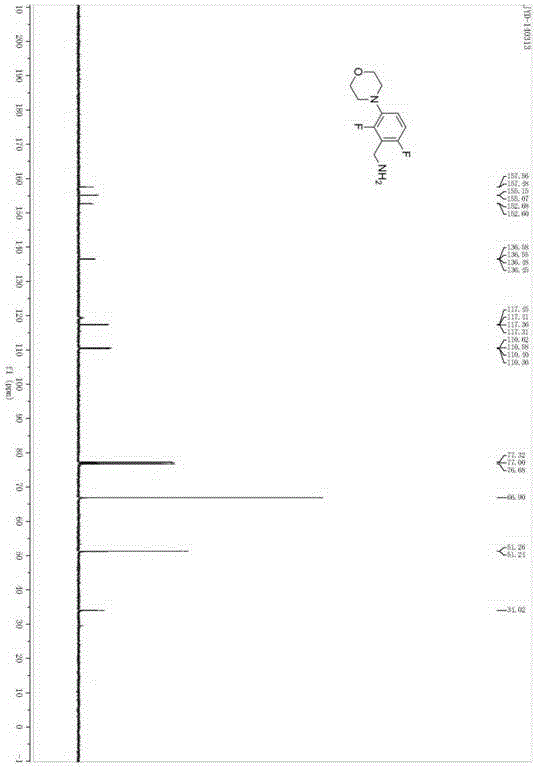
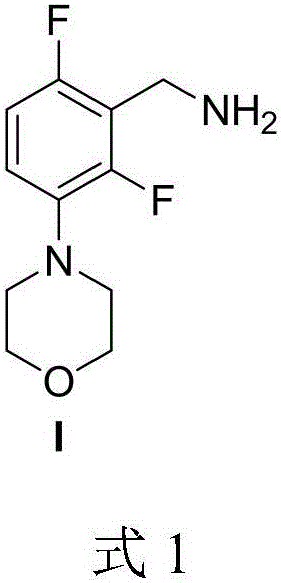
Medicine intermediate 2,6-difluoro-3-morpholinophenyl methylamine and preparation method thereof
A technology of morpholine phenylmethylamine and difluorobenzonitrile, which is applied in the field of pharmaceutical intermediate 2,6-difluoro-3-morpholine phenylmethylamine and its preparation, can solve the problems of difficult scale-up production, high cost and high production cost. Low efficiency and other problems, to achieve the effect of easy control and operation, cheap price and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A preparation method of pharmaceutical intermediate 2,6-difluoro-3-morpholine phenylmethylamine, comprising the steps of:
[0025] Dissolve 5g of 2,6-difluorobenzonitrile II in 15.0mL of concentrated sulfuric acid, add 6.0mL of concentrated nitric acid at -10°C,
[0026]
[0027] After the addition, the reaction was continued at 20°C for 5 hours. After cooling, it was extracted with dichloromethane, dried over anhydrous sodium sulfate, filtered, and evaporated to remove the solvent under reduced pressure to obtain the nitrated product III.
[0028] 2) Dissolve 6.0 g of the nitration product III obtained in the previous step in 50.0 mL of isopropanol, add 34 g of ammonium chloride aqueous solution with a mass concentration of 40%, and iron powder (6.0 g) successively, and react at a temperature of 80° C. for 10 hours.
[0029]
[0030] After cooling, the insoluble matter was removed by filtration, the organic phase was washed successively with water and saturated b...
Embodiment 2
[0038] A preparation method of a pharmaceutical intermediate 2,6-difluoro-3-morpholine phenylmethylamine, comprising the following steps, the reaction formula is shown in formula 3 to formula 6:
[0039] 1) Dissolve 7.5g of 2,6-difluorobenzonitrile II in 23.0mL of concentrated sulfuric acid, add 18.0mL of concentrated nitric acid at 0°C, continue the reaction at 25°C for 3 hours after the addition, cool with dichloro Extract with methane, dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure to obtain the nitrated product III.
[0040] 2) Dissolve 8.0 g of the nitrated product III obtained in the previous step in 80.0 mL of isopropanol, add 60 g of ammonium chloride aqueous solution and iron powder (13.0 g) with a mass ratio of 40% in sequence, and react at a temperature of 90° C. for 8 hours. After cooling, the insoluble matter was removed by filtration, the organic phase was washed successively with water and saturated brine, dried over anhy...
Embodiment 3
[0044] A preparation method of a pharmaceutical intermediate 2,6-difluoro-3-morpholine phenylmethylamine, comprising the following steps, the reaction formula is shown in formula 3 to formula 6:
[0045]1) Dissolve 10.0g of 2,6-difluorobenzonitrile II in 40.0mL of concentrated sulfuric acid, add 30.0mL of concentrated nitric acid at 5°C, continue the reaction at 35°C for 2 hours after the addition, cool with dichloro Extract with methane, dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure to obtain the nitrated product III.
[0046] 2) Dissolve 11.0 g of the nitration product III obtained in the previous step in 130.0 mL of isopropanol, add 90 g of ammonium chloride aqueous solution with a mass concentration of 40%, and iron powder (20.0 g) successively, and react at a temperature of 110° C. for 6 hours, After cooling, the insoluble matter was removed by filtration, the organic phase was washed successively with water and saturated brine, d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com