Difunctional fluorescent probe, preparation method and application
A fluorescent probe and dual-function technology, which is applied in the field of fluorescent probe preparation, can solve the problems of large effect influence, long response time, poor water solubility of the probe, etc., and achieve simple preparation process, good selectivity and specificity Sexuality and ease of large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of Fluorescent Probes for HClO Detection and Mitochondrial Localization
[0031] Dissolve 269mg of 2-(5-methyl-4-formyl-2-hydroxyphenyl)benzothiazole and 235mg of 1,4-lutidine iodide in 60ml of ethanol solution, then piperidine 1.0mMo l Add dropwise to the above solution; under the condition of oil bath, vigorously stir the reaction overnight; Filter, vacuum-dry to obtain 393mg of tan solid, be the fluorescent probe pure product of HClO detection and mitochondria localization ( 1 H-NMR chart and high-resolution mass spectrogram see Figure 7 , Figure 8 ). The measured molecular weight of the obtained pure fluorescent probe was 486.37.
[0032] Present embodiment process route:
[0033]
Embodiment 2
[0034] Example 2 Spectral properties of HClO detection and mitochondrial localized fluorescent probes reacting with various ions
[0035] Weigh 4.9 mg of fluorescent probes for HClO detection and mitochondrial localization prepared in Example 1, and prepare 10 mL of CH with a concentration of 1 mM 3 CN solution, as the mother liquor.
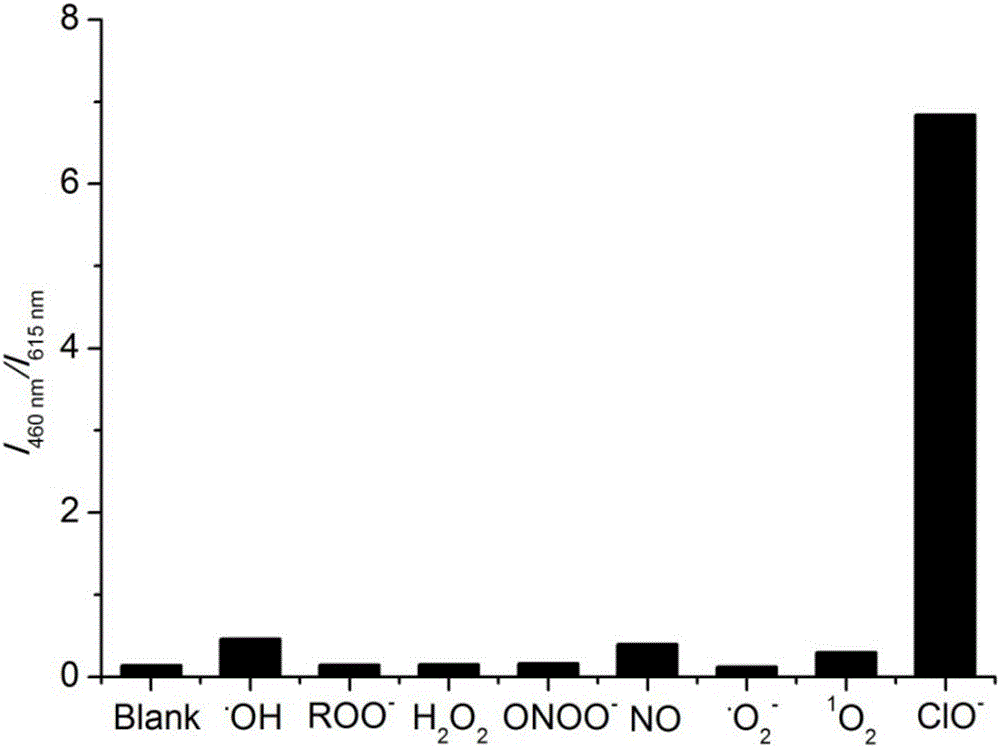
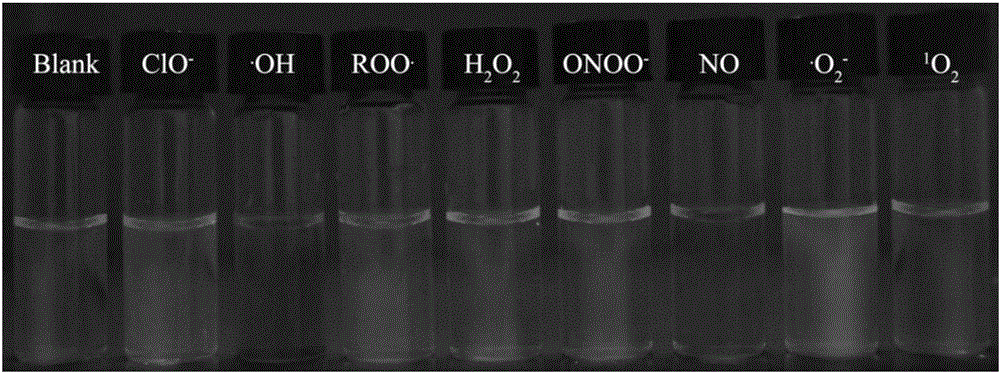
[0036] Fluorescence spectrum test: Add 30 μL of the above mother solution into a certain amount of 10mM HEPES buffer solution (pH 7.4), and then add various ions: H 2 o 2 ,NO, . o 2 - ,ONOO - ,ROO . , 1 o 2 , . OH, ClO - , so that the final concentration of ions is 500 μM, and the final concentration of fluorescent probes is 10 μM. The fluorescence emission spectrum was tested immediately under the excitation wavelength of 398nm. The slit width for excitation and emission is 5 / 10nm. Income I 460nm / I 615nm The histogram of figure 1 shown. The solution prepared above was irradiated with a 365nm ultraviolet lamp, and the fluoresce...
Embodiment 3
[0040] Example 3 HClO Detection and Mitochondrial Localization Fluorescent Probes and ClO -Spectral properties of reaction products
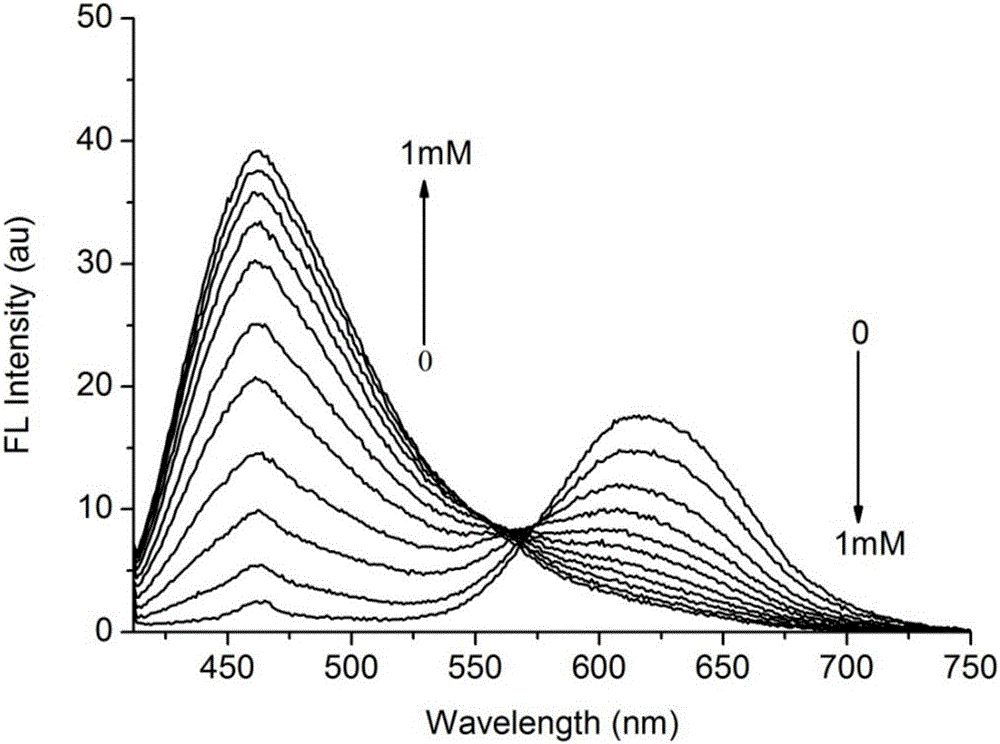
[0041] 30 μ L of the mother solution in Example 2 was added to a certain amount of 10 mM HEPES buffer solution (pH 7.4), and then different equivalents of ClO were added - , so that the final concentration of the fluorescent probe is 10 μM, and the final concentration of gold ions is 0 μM, 0.1 mM, 0.2 mM, 0.3 mM, 0.4 mM, 0.5 mM, 0.6 mM, 0.7 mM, 0.8 mM, 0.9 mM, 1.0 mM, respectively. Immediately after the addition of ions, their fluorescence emission spectra were measured. The excitation wavelength is 398nm when measuring the fluorescence emission spectrum; the slit width of excitation and emission is 5 / 10nm. The resulting fluorescence intensity I 460nm / I 615nm Incremental graph see image 3 ; with I 460nm / I 615nm The data to make the working curve, the results are shown in Figure 4 .
[0042] The experimental results show that after t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com