Polypeptide having anti-bacterial and anti-inflammatory activity and application thereof
A peptide sequence, bacterial technology, applied in the application of skin care products or cosmetics, drugs and external products such as dressings, can solve problems such as weak affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Polypeptide 1 (GW28-Br)
[0030] A polypeptide 1 (GW28-Br) is a small molecule derived from the antimicrobial peptide Centrocin, consisting of 30 amino acid residues and modified by bromination, with a molecular weight of 3321.6Da. GW28-Br is an amphiphilic, α-helical antimicrobial peptide with one polar surface and one nonpolar surface. The nucleotide sequence of the polypeptide 1 (GW28-Br) is shown in SEQ ID NO.1:
[0031] GlyTrp(Br)PheLysLysThrPheHisLysValSerHisAlaValLysSerGlyIleHisAlaGlyGlnArgGlyCysSerAlaLeuGlyPhe.
Embodiment 2
[0032] Embodiment 2: polypeptide derivative
[0033] The amino acid sequences of the six derivatives of the polypeptide 1 (GW28-Br) are as follows:
[0034] SEQ ID NO.2: GlyTrp(Br)PheLysLysThrPheHisLysValSerHisAlaValLysSerGlyIleHisAla;
[0035] SEQ ID NO. 3: SerTrp(Br)PheSerArgThrValHisAsnValGlyAsnAlaValArgLysGlyIleHisAlaGlyGlnGlyValCysSerGlyLeuGlyLeu;
[0036]SEQ ID NO.4:
[0037] GlyTrp(Br)Trp(Br)ArgArgThrValAspLysValArgAsnAlaGlyArgLysValAlaGlyPheAlaSerLysAlaCysGlyAlaLeuGlyHis;
[0038] SEQ ID NO.5: GlyTrp(Br)Trp(Br)ArgArgThrValAspLysValArgAsnAlaGlyArgLysValAla;
[0039] SEQ ID NO.6:
[0040] Trp(Br)GlyHisLysLeuArgSerSerTrp(Br)AsnLysValLysHisAlaValLysLysGlyAlaGlyTyrAlaSerGlyAlaCysArgValLeuGlyHis;
[0041] SEQ ID NO. 7: Trp(Br)GlyHisLysLeuArgSerSerTrp(Br)AsnLysValLysHisAlaValLysLys.
[0042] A further derivative is a derivative produced by the substitution of an amino acid residue in the above polypeptide 1 (SEQ ID NO.1) or polypeptide derivatives (SEQ ID NO.2-7), or th...
Embodiment 3
[0043] Embodiment 3: GW28-Br in vitro antibacterial activity evaluation
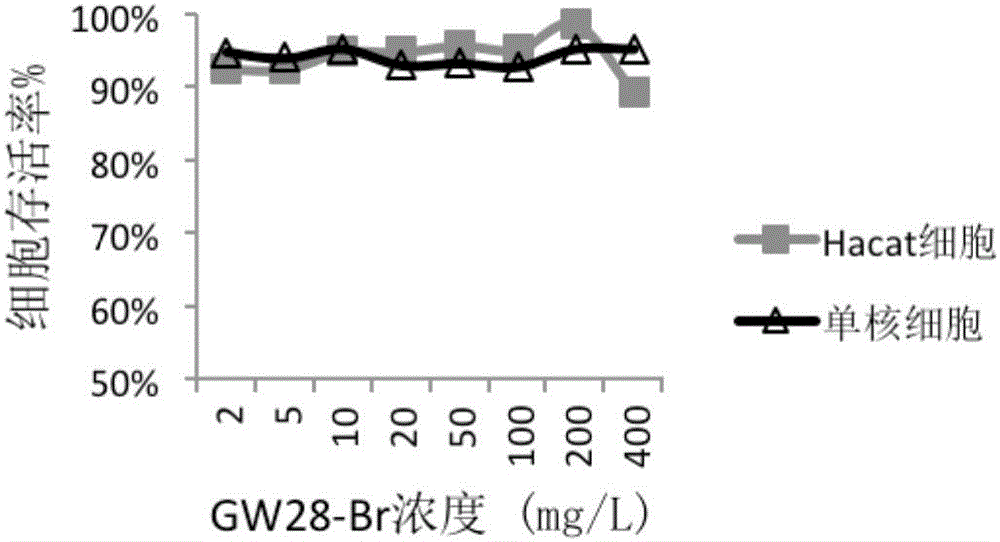
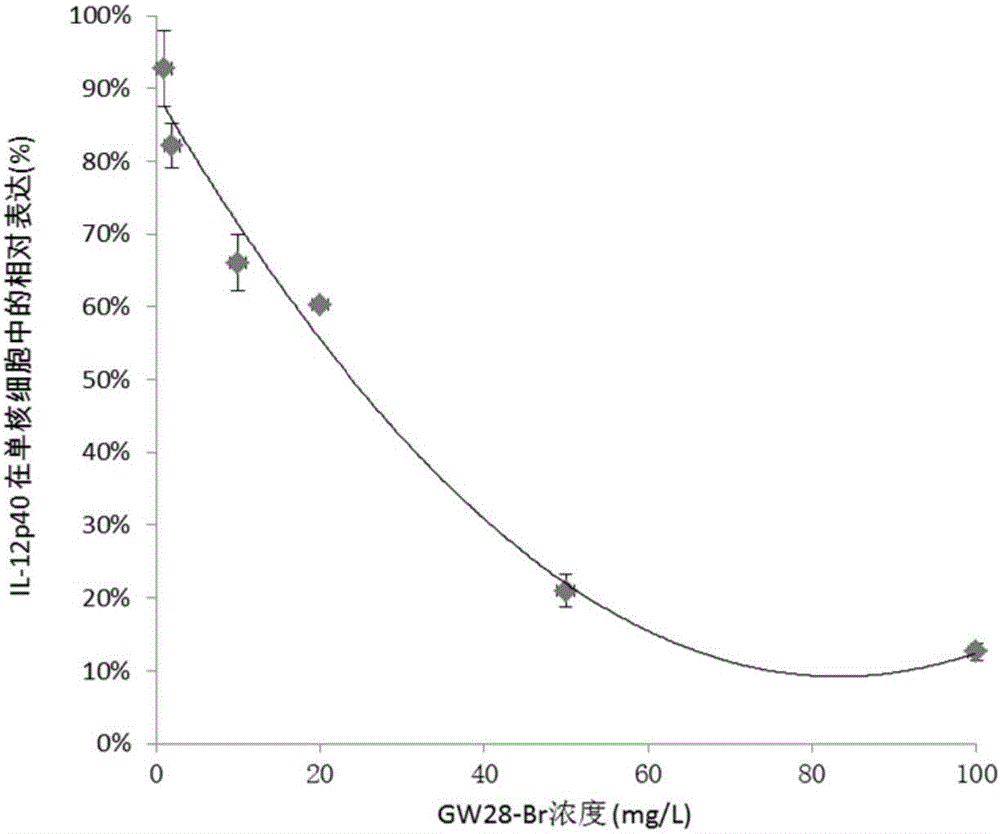
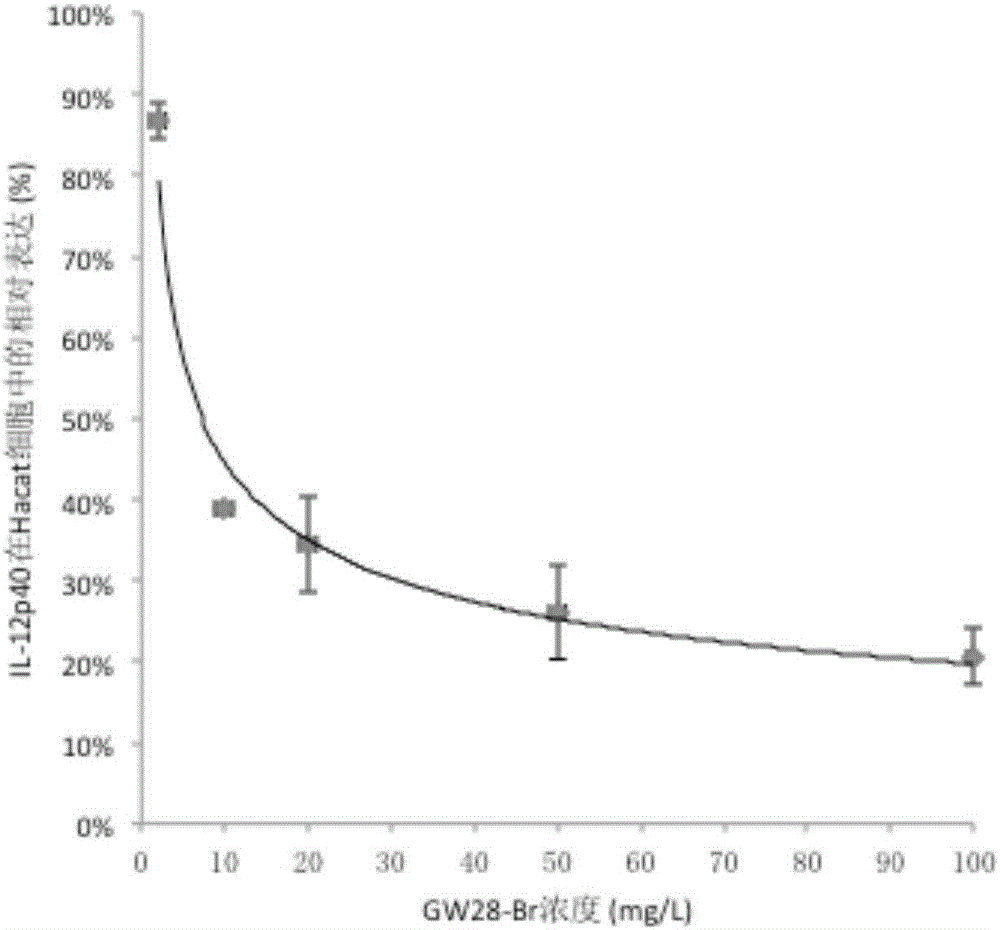
[0044] The Corynebacterium acnes isolated from the skin lesions of 15 acne patients were clinically collected, and the MIC values (minimum inhibitory concentration) of GW28-Br and clindamycin against clinical isolates and ATCC strains were detected by micro broth method. The results are shown in Table 1. GW28-Br has obvious inhibitory effect on clinically isolated Corynebacterium acnes, including 6 clindamycin-resistant strains. The average MIC value of GW28-Br was 11.8 mg / L, which was significantly lower than the MIC value of 31.2 mg / L of clindamycin against 9 clinical strains of Corynebacterium acnes (P<0.05). GW28-Br can also inhibit Staphylococcus aureus ATCC 25913 (MIC value 32 mg / L) and Staphylococcus epidermidis ATCC 12228 (MIC value 16 mg / L) commonly found in acne lesions, while clindamycin can only inhibit Staphylococcus epidermidis ATCC12228 (MIC value 64mg / L).
[0045] Table 1 MIC value (m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com