Broad-spectrum absorption macromolecule ultraviolet absorber and preparation method thereof
A technology of ultraviolet rays and macromolecules, applied in the field of macromolecular ultraviolet absorbers with broad-spectrum absorption and its preparation, can solve the problems of weakening the effect of ultraviolet light absorption, and achieve the effect of excellent broad-spectrum ultraviolet absorption function and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
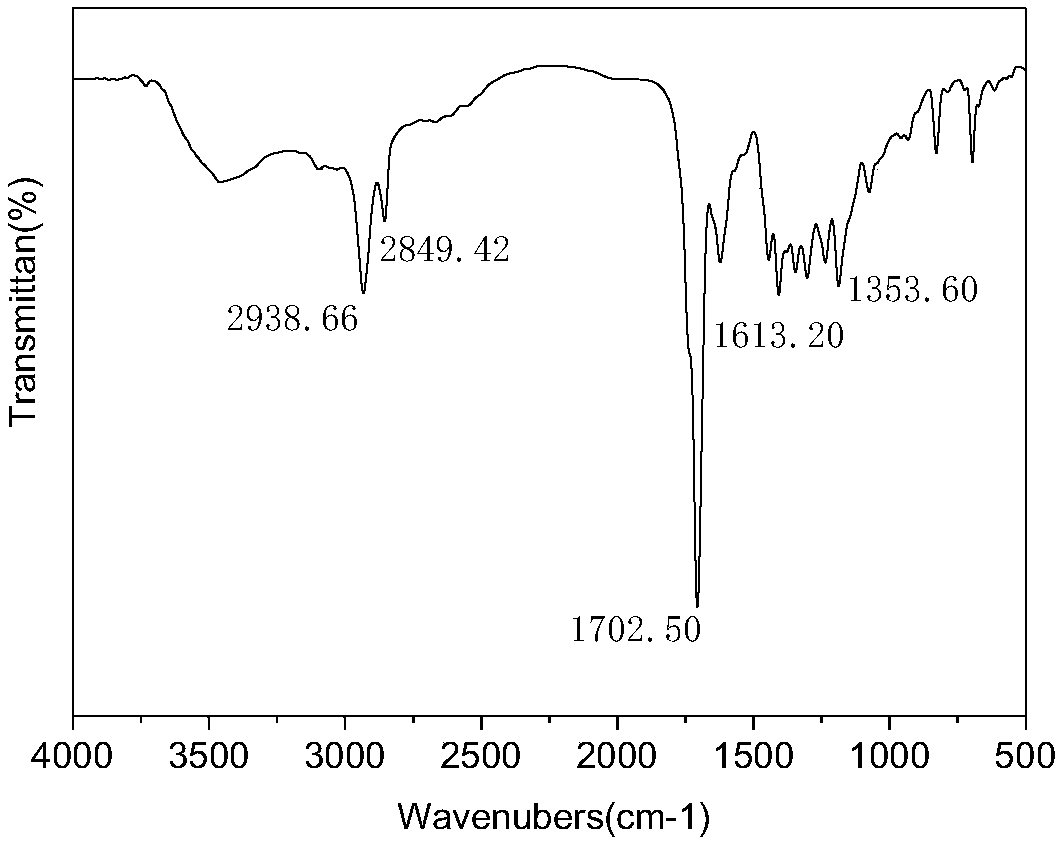
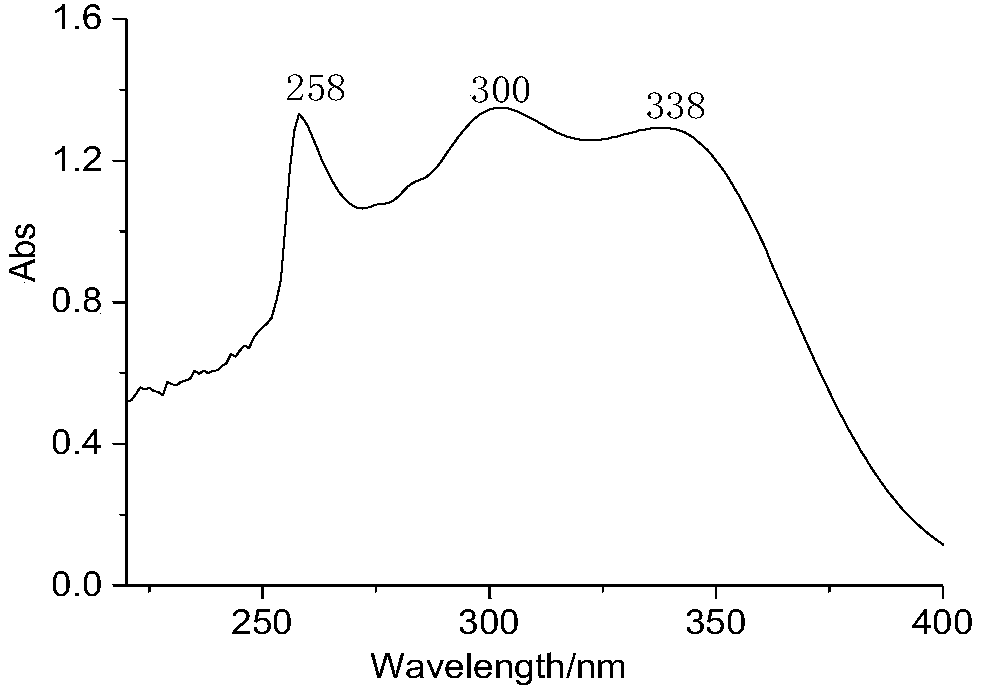
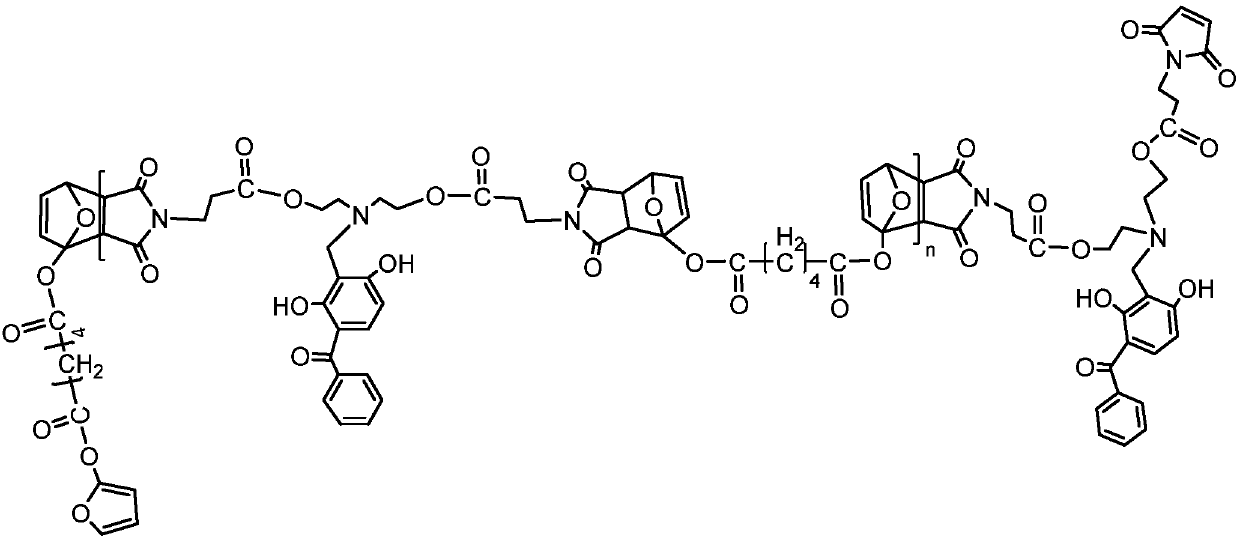
[0028] The invention provides a kind of preparation method of the macromolecule ultraviolet absorber of broad-spectrum absorption, comprising: utilizing 3-(N, N-2-maleimido ethyl propionate-aminomethyl)-2,4 - A Diels-Alder reaction of dihydroxybenzophenone with difurfuryl sebacate or adipate as a raw material monomer.
[0029] The macromolecule ultraviolet absorber prepared in the broad-spectrum absorbing macromolecular ultraviolet absorber and the preparation method thereof of the present invention has excellent broad-spectrum ultraviolet absorbing function; The chain contains heterocycles and ester groups, and has good compatibility with most polymer materials such as polystyrene, ABS, polycarbonate, polyurethane, and Diels-Alder click polymers, and is suitable for this type of high polymer. Light stabilization of molecular materials; in addition, the reaction of the macromolecular ultraviolet absorber does not require a catalyst, and the reaction conditions are mild, which ...
Embodiment 1
[0041] (1) Synthesis of 3-(N, N-2-hydroxyethyl-aminomethyl)-2,4-dihydroxybenzophenone (HAHBP)
[0042] UV-0: diethanolamine=1: 1.3 (mol); UV-0: paraformaldehyde=1: 1.5 (mol),
[0043] Put 1.365g (0.013mol) of diethanolamine into a 150mL three-necked flask, and start adding 0.45g (0.015mol) of paraformaldehyde powder in batches when heating at 60°C, and put in 0.15g of paraformaldehyde every 15 minutes, and put in 3 batches After the addition, 60 ° C insulation reaction for 1.0 h, then 2.14 g (0.01 mol) of UV-0 dissolved in 8 mL of methanol, added to the above reaction solution, continued reaction at 60 ° C for 2.5 h. Then the product is washed with water to remove excess diethanolamine and paraformaldehyde, freeze-dried to obtain the product, and its synthetic route is:
[0044]
[0045] (2) Synthesis of 3-(N,N-2-maleimido ethyl propionate-aminomethyl)-2,4-dihydroxybenzophenone (EMIPAHBP)
[0046] MIPA:HAHBP=2:1 (mol), MIPA:DCC=1:1 (mol).
[0047] In a 150mL three-necked...
Embodiment 2
[0060] (1) Synthesis of 3-(N, N-2-hydroxyethyl-aminomethyl)-2,4-dihydroxybenzophenone (HAHBP)
[0061] UV-0: diethanolamine=1: 2 (mol): UV-0: paraformaldehyde=1: 1.5 (mol),
[0062] Put 2.23g (0.0212mol) of diethanolamine into a 150mL three-necked flask, and start adding paraformaldehyde powder in batches at 70°C, a total of 0.477g (0.0159), and put in 0.159g of paraformaldehyde every 15min, and put it in 3 batches. After the addition, the temperature was raised to 75°C for 2.5 hours, and then 2.27g (0.0106mol) of UV-0 was dissolved in 10mL of methanol, added to the above reaction solution, and the reaction was continued at 75°C for 5h. Then the product is washed with water to remove excess diethanolamine and paraformaldehyde, freeze-dried, and the synthetic route is:
[0063]
[0064] (2) Synthesis of 3-(N,N-2-maleimido ethyl propionate-aminomethyl)-2,4-dihydroxybenzophenone (EMIPAHBP)
[0065] MIPA:HAHBP=2.5:1, MIPA:DCC=1:1 (mol).
[0066] In a 150mL three-necked flask...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com