Synthesis method for Cu-N-C catalyst by hydrothermal method
A catalyst and hydrothermal technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of low ORR activity, incomplete four-electron transfer, etc., and achieve simple preparation process, adjustable catalytic performance, and low equipment requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
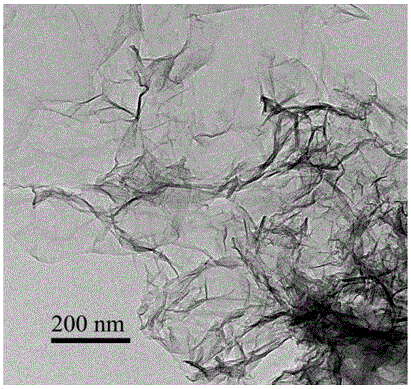
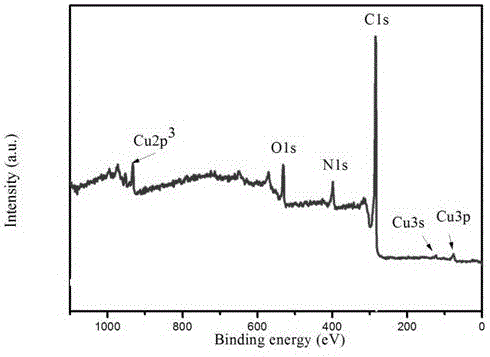
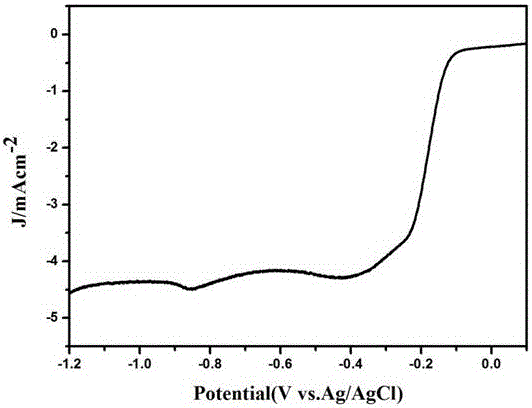
Image
Examples
Embodiment 1
[0025] (1) First, grind the copper block with a purity of 99.99% into copper powder with a particle size of 10-50 μm, then weigh 30 mg of copper powder and put it into a 20 mL glass bottle, then add 15 mL of ammonia (NH 3 ) ammonia water with a concentration of 25%, until the colorless transparent liquid in the glass bottle turns into a light blue liquid; seal the light blue liquid and put it in the refrigerator at 4°C, after 4 days, the light blue The liquid becomes a colorless and transparent liquid again. After removing the copper residue in the colorless and transparent liquid in a glove box under anaerobic conditions, Cu[NH] containing monovalent copper ions (Cu(I)) is obtained. 3 ] 2 OH is a colorless transparent liquid, called Cu(I) liquid;
[0026] (2) Take 100mg of graphene oxide and prepare 20mL of graphene oxide distilled solution with a concentration of 5mg / mL (ultrasound for 30min), mix it with the Cu(I) liquid in step (1) and put it into a Teflon-lined container...
Embodiment 2
[0029] (1) First, grind the copper block with a purity of 99.99% into copper powder with a particle size of 50-100 μm, then weigh 22.5 mg of copper powder and put it into a 20 mL glass bottle, then add 15 mL of ammonia (NH 3) content of 30% ammonia water, until the colorless transparent liquid in the glass bottle turns into a light blue liquid. Put the light blue liquid into a refrigerator at 4°C after sealing it well, and after standing for 3 days, the light blue liquid becomes a colorless and transparent liquid again. After removing copper residues in a colorless transparent liquid in a glove box under anaerobic conditions, Cu[NH] containing monovalent copper ions (Cu(I)) 3 ] 2 OH is a colorless and transparent liquid, called Cu(I) liquid; Cu(I) liquid is placed in the air for 10 minutes, and after the colorless and transparent liquid turns blue, the solution containing divalent copper ions (Cu(II) ) Cu[NH 3 ] 4 (OH) 2 Light blue liquid, called Cu(II) liquid;
[0030] ...
Embodiment 3
[0032] (1) First, grind the copper block with a purity of 99.99% into copper powder with a particle size of 10-100 μm, then weigh 20 mg of copper powder and put it into a 20 mL glass bottle, then add 15 mL of ammonia (NH 3 ) ammonia water with a content of 15%, the colorless transparent liquid in the glass bottle turns into a light blue liquid; after sealing the light blue liquid, put it in the refrigerator at 10°C, and after 3 days, the light blue The liquid becomes a colorless and transparent liquid again; after removing the copper residue in the colorless and transparent liquid in a glove box under anaerobic conditions, Cu[NH] containing monovalent copper ions (Cu(I)) is obtained 3 ] 2 OH is a colorless transparent liquid called Cu(I) liquid;
[0033] (2) Take 30 mL of graphene oxide nanoribbon distilled aqueous solution (prepared by ultrasonication for 40 min) with a concentration of 1 mg / mL, mix it with the Cu(I) liquid in step (1), and put it into a stainless steel high...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com