Low temperature injectable acrylic resin bone cement and its preparation method
An acrylic resin and bone cement technology, applied in the field of medical biomaterials, can solve problems such as changes, lowering the maximum temperature, etc., to achieve the effects of reducing the exothermic temperature, reducing the residual monomer content, and reducing the risk of complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
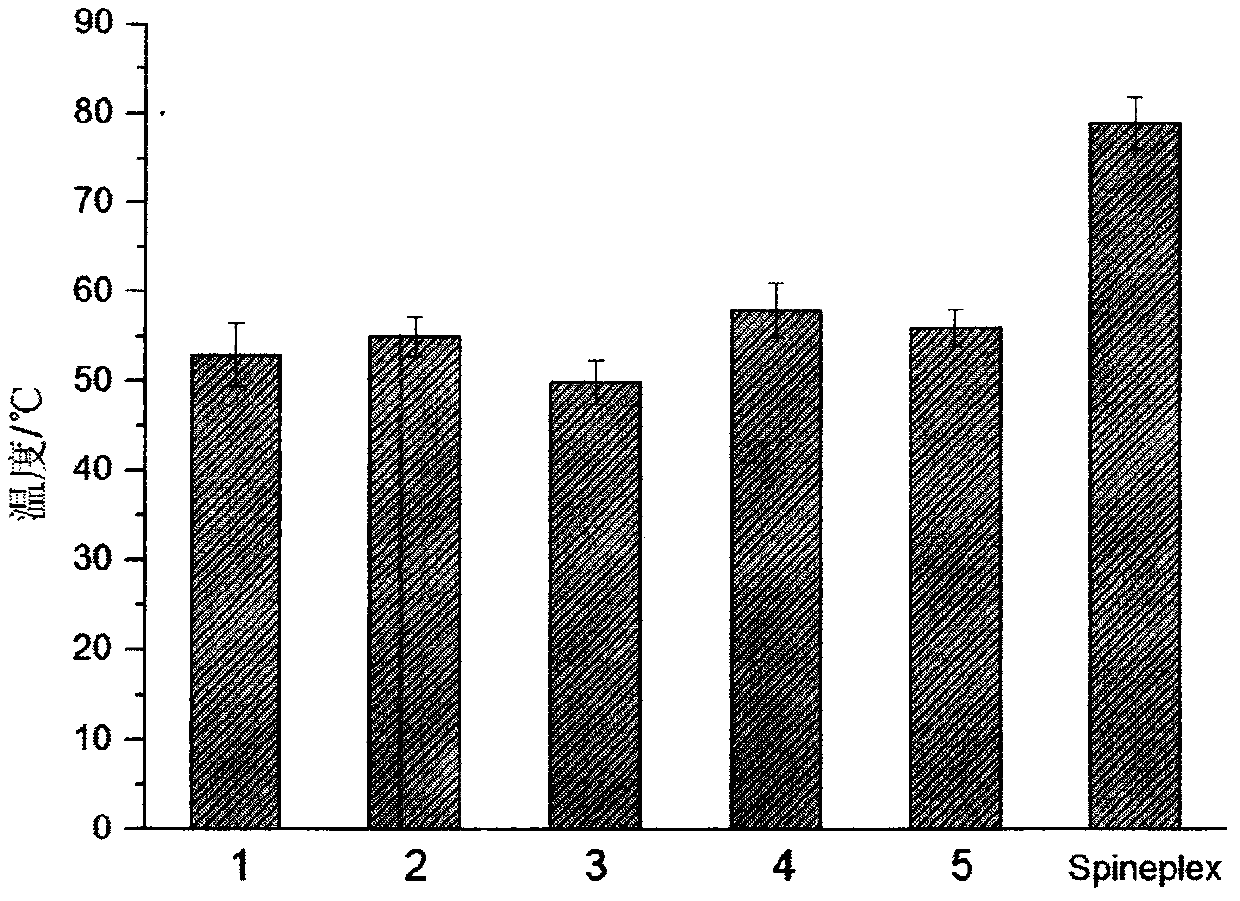
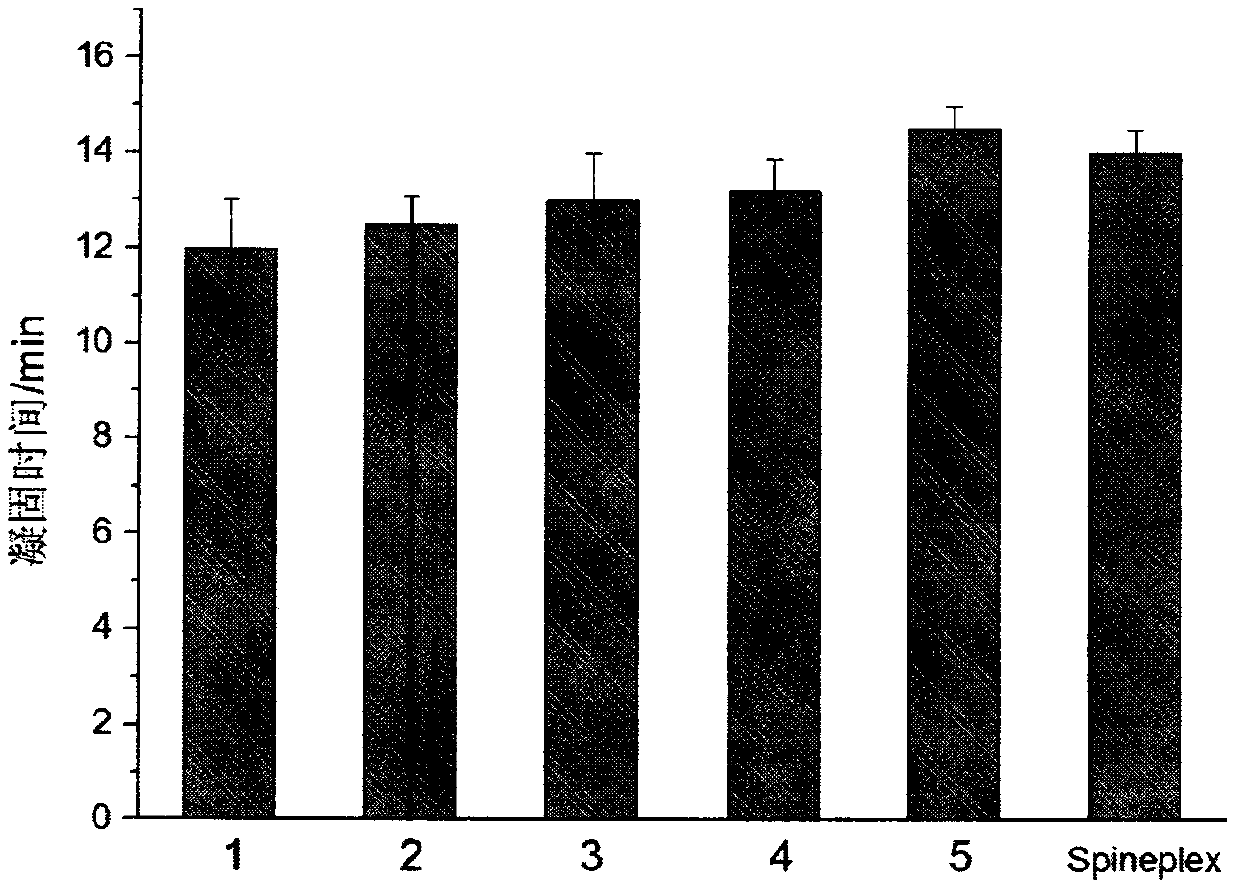
Examples
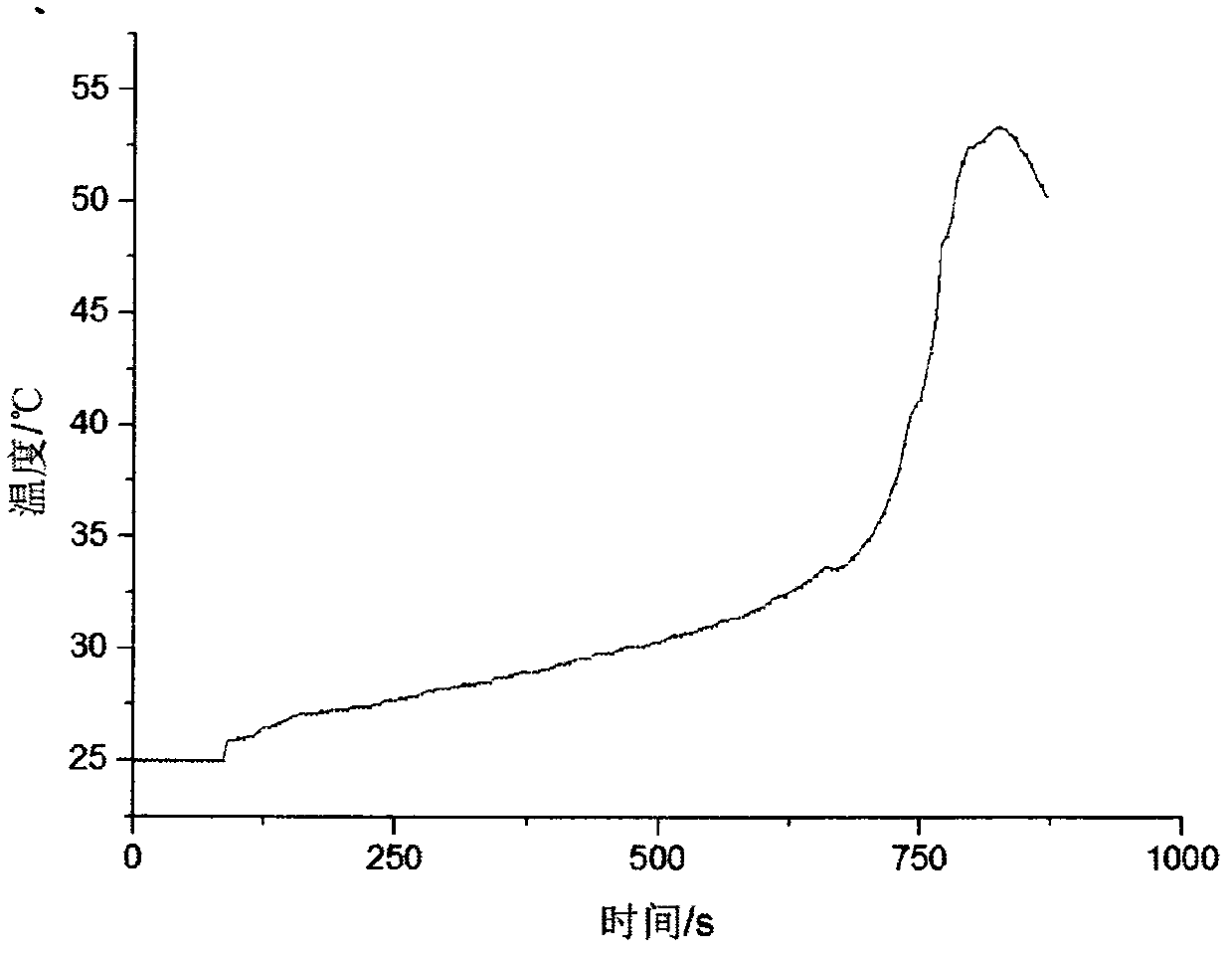
Embodiment 1
[0044] The preparation method of low temperature injectable acrylic resin bone cement comprises the following steps:
[0045] (1) Preparation of Component A
[0046] a. adopting the suspension polymerization method to prepare polymethyl methacrylate with a particle size range of 60-120 μm and a styrene-methyl methacrylate copolymer with a particle size range of 80-150 μm;
[0047] b. In terms of mass percentage, the polymethyl methacrylate prepared in 50wt% step a, the styrene-methyl methacrylate copolymer prepared in 25wt% step a, the tertiary benzoyl peroxide of 0.8wt% Butyl ester and 24.2wt% of barium sulfate with a particle size range of 30-50 μm are uniformly mixed to obtain component A;
[0048] (2) Preparation of liquid phase
[0049] c. In terms of volume percentage, mix 99vol% methyl acrylate and 1vol% N-N-dimethyl-p-toluidine evenly, and then add 20ppm hydroquinone to obtain a liquid phase;
[0050] (3) Preparation of Component B
[0051] d. Add the liquid phase ...
Embodiment 2
[0064] The preparation method of low temperature injectable acrylic resin bone cement comprises the following steps:
[0065] (1) Preparation of Component A
[0066] a. adopting the suspension polymerization method to prepare polymethyl methacrylate with a particle size range of 20-100 μm and a styrene-methyl methacrylate copolymer with a particle size range of 100-200 μm;
[0067] b. In terms of mass percentage, the polymethyl methacrylate prepared in 30wt% step a, the styrene-methyl methacrylate copolymer prepared in 29.5wt% step a, the tertiary benzoyl peroxide of 2wt% Butyl ester and 38.5wt% of barium sulfate with a particle size range of 1-20 μm are uniformly mixed to obtain component A;
[0068] (2) Preparation of liquid phase
[0069] c. In terms of volume percentage, mix 95vol% butyl methacrylate and 5vol% N-N-dimethyl-p-toluidine evenly, and then add 80ppm hydroquinone monomethyl ether to obtain a liquid phase;
[0070] (3) Preparation of Component B
[0071] d. A...
Embodiment 3
[0078] The preparation method of low temperature injectable acrylic resin bone cement comprises the following steps:
[0079] Preparation of Component A
[0080] a. adopting the suspension polymerization method to prepare polymethyl methacrylate with a particle size range of 20-80 μm and a methyl acrylate-methyl methacrylate copolymer with a particle size range of 80-150 μm;
[0081] b. In terms of mass percentage, the polymethyl methacrylate prepared in 45wt% step a, the methyl acrylate-methyl methacrylate copolymer prepared in 44wt% step a, the tertiary benzoyl peroxide Component A is obtained by uniformly mixing butyl ester and 10 wt% of zirconia with a particle size range of 10-30 μm;
[0082] (2) Preparation of liquid phase
[0083] c. In terms of volume percentage, mix 98vol% methyl acrylate and 2vol% N-N-dimethyl-p-toluidine evenly, and then add 60ppm hydroquinone to obtain a liquid phase;
[0084] (3) Preparation of Component B
[0085] d. Add the liquid phase prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com