Compound separated out from labiatae isodon plant, and preparation method and application thereof
A technology of the genus and compound of Camellia genus, which is applied in the field of medicine, can solve problems such as unclear relationships, and achieve the effect of strong pharmacological effects and good medicinal prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
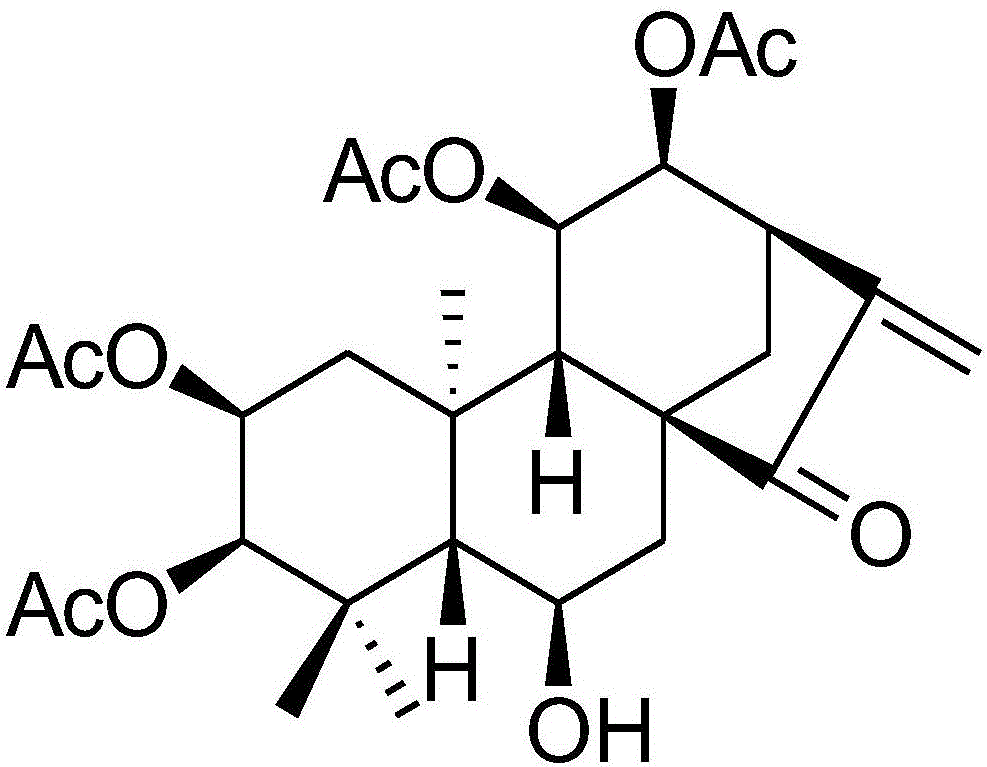
[0042] The preparation method of embodiment 1 compound Iso A
[0043] Take 10Kg of the aerial part of the plant Isodon forrestii var.forrestii and pulverize it, soak it with 70% (acetone: water) acetone water at room temperature for three times, a total of 12 liters, evaporate the acetone by distillation under reduced pressure, and extract the remaining part with ethyl acetate to obtain ethyl acetate The extract was subjected to silica gel column chromatography, 200 mesh pore size silica gel, chloroform: acetone (volume ratio 1:0.11) solvent washing, and repeated treatment with a mixed solution of 85% ethyl methanol and water through RP-18 volume ratio, and finally obtained Iso A500mg , The yield is about 0.005%.
[0044] X-single crystal of compound Iso A and physical and chemical data such as UV, optical rotation, infrared, NMR:
[0045] Crystal data for Sze1:C 28 h 38 o 10 , M=534.58, orthorhombic, α=90.00°, β=90.00°, γ=90.00°, T=100(2)K, space group P212121, Z=4,...
Embodiment 2
[0047] The preparation method of embodiment 2 compound Iso A
[0048] Take 10Kg of the aerial part of the plant Isodon forrestii var.forrestii and pulverize it, soak it three times with 80% (acetone: water) acetone water at room temperature, a total of 20 liters, evaporate the acetone by distillation under reduced pressure, and extract the remaining part with ethyl acetate to obtain ethyl acetate The extract was subjected to silica gel column chromatography, 300 mesh pore size silica gel, chloroform: acetone (1:0.5) solvent washing, and repeated treatment with RP-18 and a mixed solution of 85% acetonitrile and water by volume to finally obtain Iso A 400mg, The yield is about 0.004%.
Embodiment 3
[0049] Example 3 Iso A can selectively inhibit the growth of human tumor cells and induce apoptosis
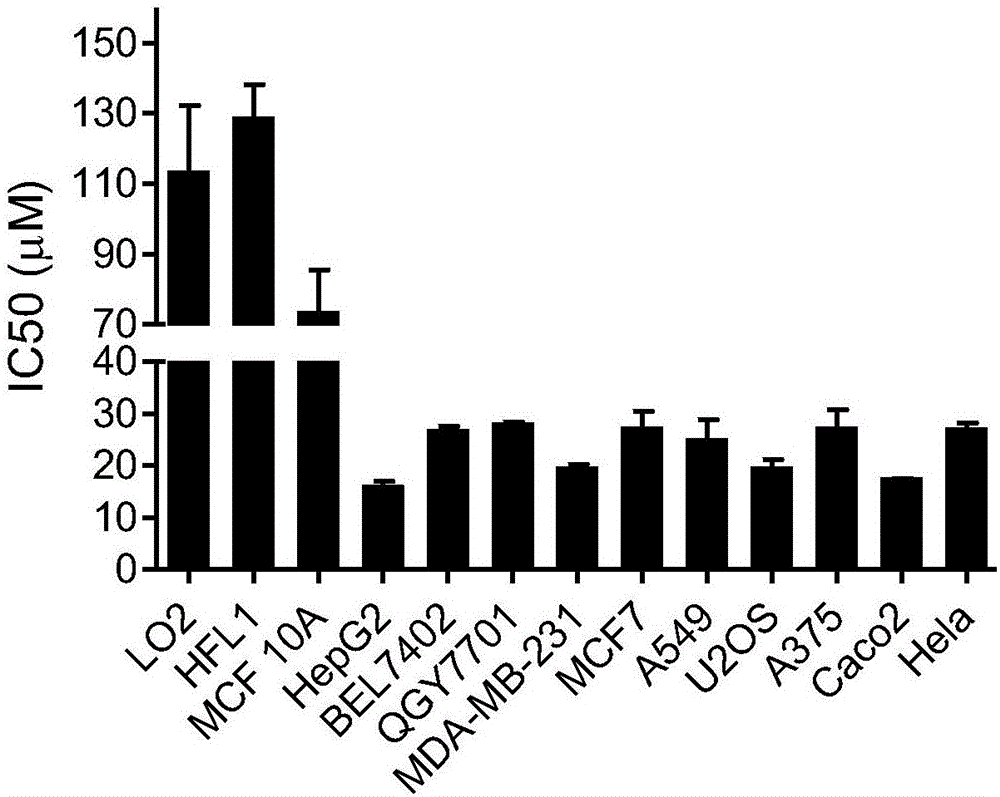
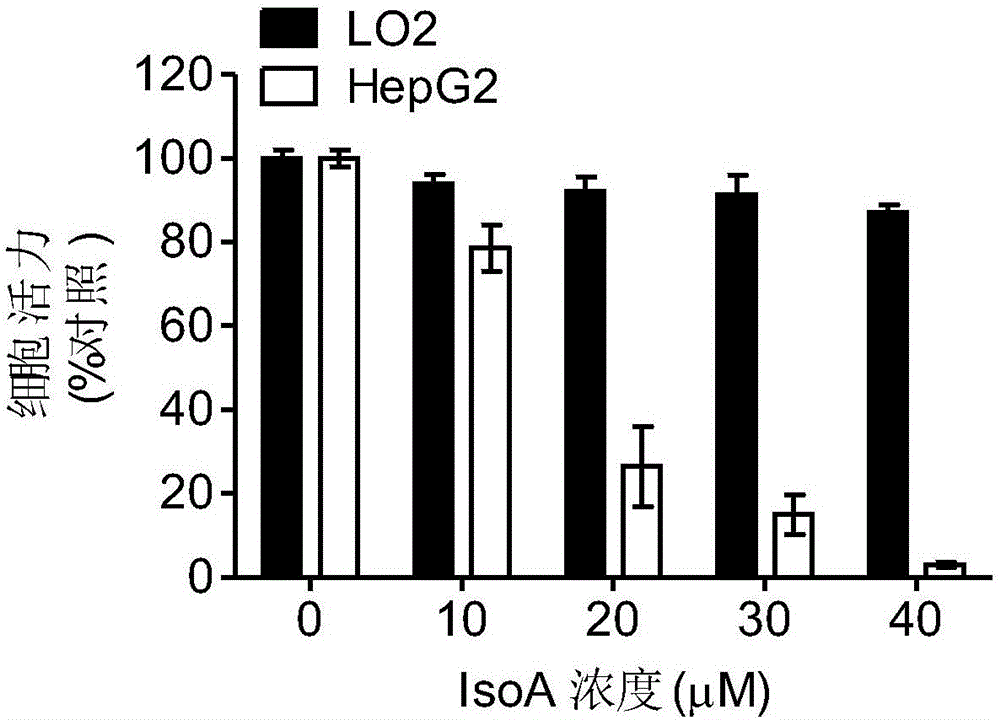
[0050] Iso A (purity>99%) was dissolved in DMSO, the stock solution concentration was 40mM, and stored at -20°C. Using MTT method to detect IsoA in a variety of tumor cells, including liver cancer, breast cancer, lung cancer, osteosarcoma, melanoma, cervical cancer, etc., as well as normal cells human liver cell LO2, human embryonic lung cell HFL1, human breast epithelial cell MCF 10A Inhibitory effect. Compared with normal cells, Iso A has a stronger inhibitory effect on tumor cells, and its IC50 values are all less than 30 μM ( figure 2 ). Among the tested cells, HepG2 cells are the most sensitive to Iso A, so HepG2 was chosen as the model for subsequent experiments. MTT assay showed that Iso A could inhibit the growth of HepG2 cells in a concentration-dependent manner, but had no significant inhibitory effect on LO2 cells ( image 3 ). Further trypan blue experiment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com