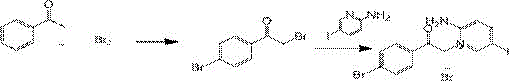Preparation method of pyridinium 2-animo-5-iodo-1-(2-(4-bromophenyl)-2-oxoethyl)bromide
A technology of pyridinium bromide and oxoethyl, applied in the field of organic synthesis, can solve problems such as high market price, difficult synthesis, lack of literature and patent reports, and achieve the effects of stable product quality, simple post-processing, and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve 600mmol (72.0g) of acetophenone in 500mL of dichloromethane, add 2mol of liquid bromine (320g) dropwise at 0°C, and stir for 3 hours after dropping. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain 2,4-dibromoacetophenone. Add 1000 mL N,N-dimethylformamide and stir to dissolve. Add 2mol 2-amino-5-iodopyridine (440g), react at 50°C for 10 hours, after the reaction is completed, cool to room temperature, add 600 mL of water and 500 mL of ethyl acetate for extraction, separate the organic phase, and use ethyl acetate for the aqueous phase Extract (3×500 mL), combine the organic phases, wash with water (2×150 mL), wash with 200 mL saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to obtain 2-amino-5-iodo-1- (2-(4-bromophenyl)-2-oxoethyl)pyridinium bromide crude product, the crude product was recrystallized from a mixed solution of n-hexane:ethyl acetate=1:1 (volume ratio) to obt...
Embodiment 2
[0023] 600mmol (72g) acetophenone, dissolved in 1000mL acetonitrile. 2mol liquid bromine (320g) was added dropwise at 0°C. Then react at 20° C. for 6 hours, and remove acetonitrile by rotary evaporation at the end of the reaction to obtain 2,4-dibromoacetophenone. Add 1200 mL of dichloromethane, add 4mol 2-amino-5-iodopyridine (880g), react at 30°C for 15 hours, after the reaction is completed, cool to room temperature, add 600 mL of water and 800 mL of ethyl acetate for extraction, separate the organic phase, The aqueous phase was extracted with ethyl acetate (3×600 mL), the organic phases were combined, washed with water (3×250 mL), washed with 300 mL saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain 2-amino- 5-iodo-1-(2-(4-bromophenyl)-2-oxoethyl)pyridinium bromide crude product, the crude product is hexane:ethyl acetate=1:2 (volume ratio) The mixed solution was recrystallized to obtain 262.9 g of pure product, wit...
Embodiment 3
[0025] 60mmol (7.2g) of acetophenone, dissolved in 250ml of chloroform. 480 mmol (76.8 g) of liquid bromine was added dropwise at 20°C. The reaction was carried out at 20°C for 5 hours. After the reaction was completed, the solvent was removed by rotary evaporation, 120 mL of acetonitrile was added, and 300 mmol (66.0 g) of 2-amino-5-iodopyridine was reacted at 10°C for 8 hours. After the reaction was completed, 60 mL of water and 60 mL of ethyl acetate were added for extraction, the organic phase was separated, and the aqueous phase was extracted with ethyl acetate (4×20 mL), the combined organic phases were washed with water (2×50 mL), and 100 mL of saturated Wash with brine, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to obtain crude product of 2-amino-5-iodo-1-(2-(4-bromophenyl)-2-oxoethyl)pyridinium bromide , the crude product was recrystallized from a mixed solution of n-hexane:ethyl acetate=1:1 (volume ratio) to obtain 28.4g of pure product,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com