Preparation method of high-purity anhydrous manganese chloride particles
An anhydrous manganese chloride and manganese chloride technology, applied in manganese halide and other directions, can solve the problem of difficult to achieve special chemical products and pharmaceutical production, difficult to prepare high-purity anhydrous manganese chloride particles, and poor dry and wet uniformity. Activity and other issues, to achieve the effect of easy industrial scale production, low cost and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
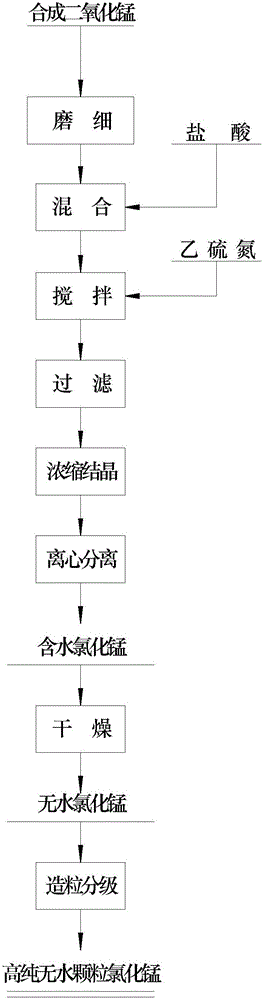
[0029] refer to figure 1 , a preparation method of high-purity anhydrous manganese chloride particles, comprising the following steps:
[0030] (1) Grinding the manganese dioxide finely;
[0031] (2) Mixing the finely ground manganese dioxide with hydrochloric acid, stirring and reacting to obtain a manganese chloride solution;
[0032] (3) adding ethylsulfide nitrogen to the manganese chloride solution;
[0033] (4) filtering the solution obtained in step (3) to obtain a filtrate;
[0034] (5) Concentrating and crystallizing the filtrate to obtain crystal slurry;
[0035] (6) centrifuging the crystal slurry to obtain hydrated manganese chloride;
[0036] (7) drying the hydrated manganese chloride to obtain anhydrous manganese chloride;
[0037] (8) Granulating the anhydrous manganese chloride to obtain granular anhydrous manganese chloride;
[0038] (9) Classifying the granular anhydrous manganese chloride to obtain high-purity anhydrous granular manganese chloride with...
Embodiment 1
[0040] Synthetic manganese dioxide is selected as the raw material, and the synthetic manganese dioxide is ground until 90% of it passes through 100 mesh. The finer particle size is finer and easier to react fully. Dilute hydrochloric acid to a concentration of 20% in advance, then mix finely ground synthetic manganese dioxide and diluted hydrochloric acid at a mass ratio of 1:1.6, stir after mixing to react to obtain a manganese chloride solution. Add ethoxynitrogen to the manganese chloride solution, add ethoxynitrogen 0.2 g per liter of the solution, and filter the solution after the reaction is completed to obtain a filtrate. Concentrating and crystallizing the filtrate to obtain a magma; centrifuging the magma to obtain hydrous manganese chloride with a water content of 7%. The hydrated manganese chloride is dried at a temperature of 250° C. to obtain anhydrous manganese chloride. The anhydrous manganese chloride is granulated on a disc pelletizer, and the granulation ti...
Embodiment 2
[0042] Synthetic manganese dioxide is selected as the raw material, and the synthetic manganese dioxide is ground until 90% of it passes through 100 mesh, and the finer particle size is finer and easier to react fully. Dilute hydrochloric acid to a concentration of 20% in advance, then mix finely ground synthetic manganese dioxide and diluted hydrochloric acid at a mass ratio of 1:1.6, stir after mixing to react to obtain a manganese chloride solution. Add ethoxynitrogen to the manganese chloride solution, add ethoxynitrogen 0.3 g per liter of solution, and filter the solution after the reaction is completed to obtain a filtrate. Concentrating and crystallizing the filtrate to obtain a magma; centrifuging the magma to obtain hydrated manganese chloride with a water content of 8%. Dry the hydrated manganese chloride at a temperature of 280° C. to obtain anhydrous manganese chloride. The anhydrous manganese chloride is granulated on a disc pelletizer, and the granulation time i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com