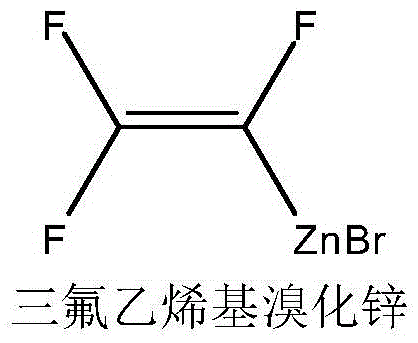
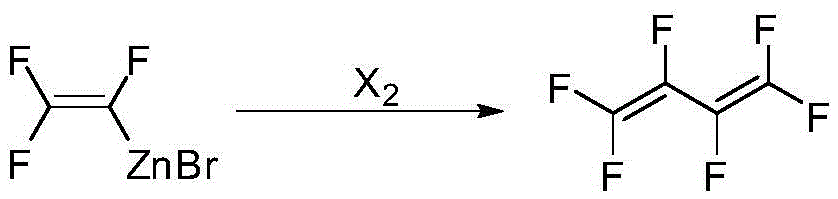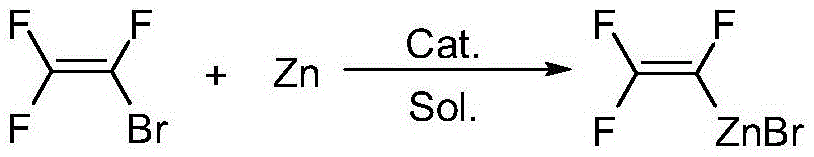Preparation method of hexafluoro-1,3-butadiene
A technology of butadiene and bromotrifluoroethylene, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, zinc organic compounds, etc., can solve the problems of high flushing temperature, decomposition of brominated trifluoroethylene zinc reagent, and large amount of three wastes, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: preparation of zinc reagent
[0031] Add N,N-dimethylformamide (600g, 8.2mol), zinc powder 85.0g (1.3mol), 1,2-dibromoethane 5.6g (0.03 mol), the temperature was lowered to 0°C with mechanical stirring, and 161 g (1.0 mol) of bromotrifluoroethylene was slowly introduced. After the reaction was initiated, the temperature was kept at 40°C until the bromotrifluoroethylene in the reaction solution was completely converted, and the stirring was stopped. After cooling down to room temperature, the The excess zinc powder is separated to obtain a zinc reagent solution. use 19 Quantification by FNMR (trifluorotoluene as internal standard), the reaction yield was 88.4%.
[0032] Zinc reagent and internal standard NMR fluorine spectrum data, 19 FNMR (CDCl 3 ,376MHz): δ-86.80(s, trifluorotoluene peak, 3F), -119.63(dd, J 1 =32Hz,J 2 =92Hz, 1F), -153.74(dd, J 1 =J 2 =32Hz, 1F), -217.99(dd, J 1 =32Hz,J 2 = 103Hz, 1F).
Embodiment 2
[0033] Embodiment 2: preparation of zinc reagent
[0034] Add 1,3-dimethyl-2-imidazolidinone (600g, 5.26mol), 84.5g (1.3mol) of zinc powder, 1,2-dibromoethane 5.64g (0.03mol), mechanically stirred and cooled down to 0°C, slowly passed into 161g (1.0mol) of bromotrifluoroethylene, and kept warm at 40°C after the reaction was triggered, until the bromotrifluoroethylene in the reaction solution was completely converted, stopped stirring, and dropped to 0°C. After reaching room temperature, the excess zinc powder was separated to obtain a zinc reagent solution. use 19 FNMR quantification (trifluorotoluene as internal standard), the reaction yield was 82.1%.
Embodiment 3
[0035] Embodiment 3: preparation of zinc reagent
[0036]Add N,N-dimethylacetamide (600g, 6.89mol), zinc powder 84.5g (1.3mol), trifluorovinyl zinc bromide mother liquor 46.2g ( Actual trifluorovinyl zinc bromide 11.3g, 0.05mol, Example 1), mechanically stirred and lowered the temperature to 0°C, slowly introduced 161g (1.0mol) of trifluoroethylene bromide, and kept warm at 40°C after the reaction was triggered, until the reaction liquid The conversion of bromotrifluoroethylene is complete. Stirring was stopped, and the excess zinc powder was separated after cooling down to room temperature to obtain a zinc reagent solution. use 19 FNMR quantification (trifluorotoluene as internal standard), the reaction yield was 86.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com