Preparation method of aryl substituted biphenyl, pyrrole, indole or binaphthyl monophosphine ligand
A binaphthyl mono- and phenylindole-type technology, applied in chemical instruments and methods, phosphorus organic compounds, organic chemistry, etc., can solve the problems of low overall yield, affecting the development and application of ligands, and single structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
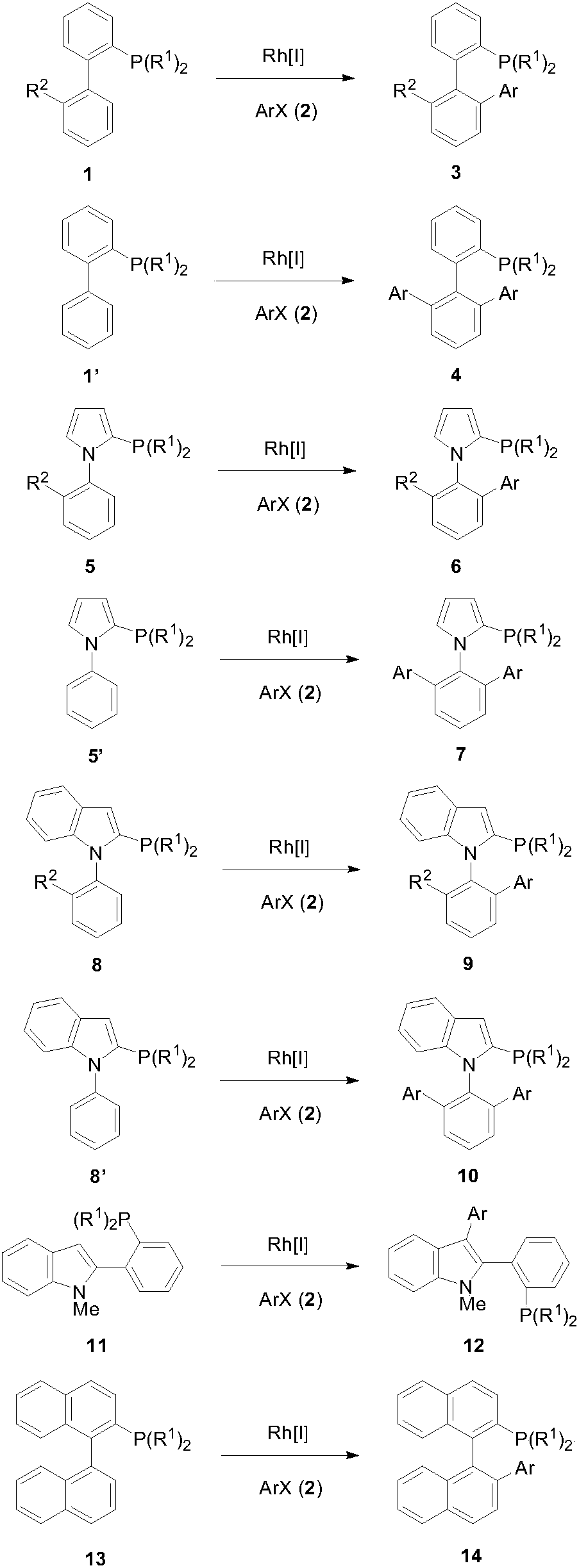
[0023] The following are specific embodiments of the present invention, further describing the technical solution of the present invention, but the present invention is not limited to this embodiment.
[0024] (1) Synthesis of 2-dicyclohexylphosphine-2'-phenylbiphenyl
[0025] Method 1: In a 25mL Schlenk tube, add 2-dicyclohexylphosphine biphenyl (CyJohnPhos) 70.1mg (0.2mmol), (1,5-cyclooctadiene) chloride rhodium (I) dimer 2.5mg (raw material mole 2.5% of the number), 48mg (0.6mmol) of lithium tert-butoxide, and replaced the argon three times, and added 1mL of 1,4-dioxane and 31.4mg (0.2mmol) of bromobenzene under the protection of argon. Stir at 70°C for 36 hours, cool to room temperature, evaporate the solvent under reduced pressure, separate through a 200-300 mesh silica gel chromatography column, elute with petroleum ether: ethyl acetate = 100:1, and obtain 73.5 mg of viscous product after vacuum drying , yield 86%.
[0026] Method 2: In a 25mL Schlenk tube, add 70.1mg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com