Polyether glycol, preparation method thereof, and foam material
A polyether polyol and reaction technology, applied in the field of polyol compounds, can solve problems such as poor flame retardancy, and achieve the effects of good flame retardancy, shortened foaming time, and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
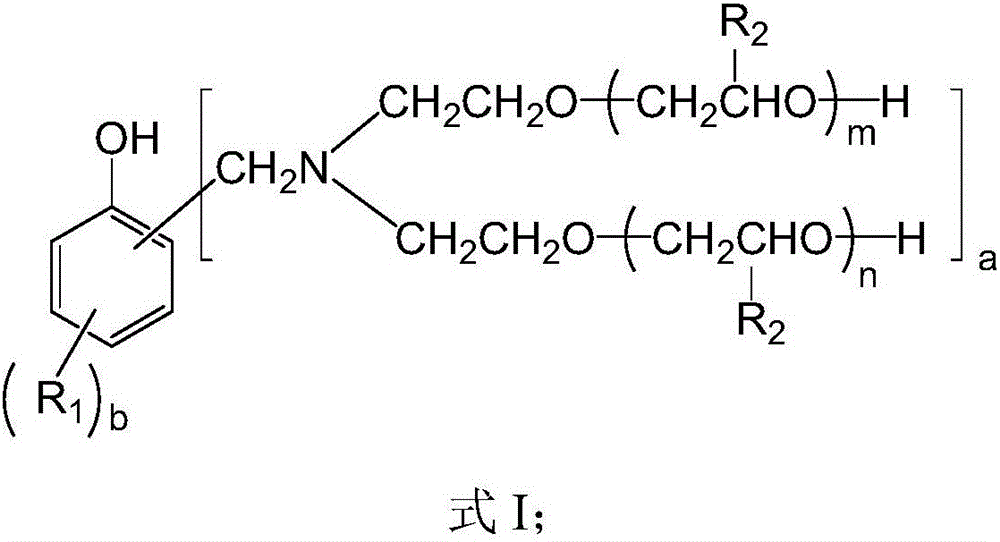
[0044] The preparation method of polyether polyol of the present invention may further comprise the steps:
[0045] Provide N-hydroxyethyl oxazolidine;
[0046] Mannich bases are provided, said Mannich bases are subjected to Mannich reactions from unsubstituted or alkyl substituted phenols and N-hydroxyethyloxazolidine;
[0047] And, ring-opening addition of the Mannich base and unsubstituted or alkyl-substituted oxirane.
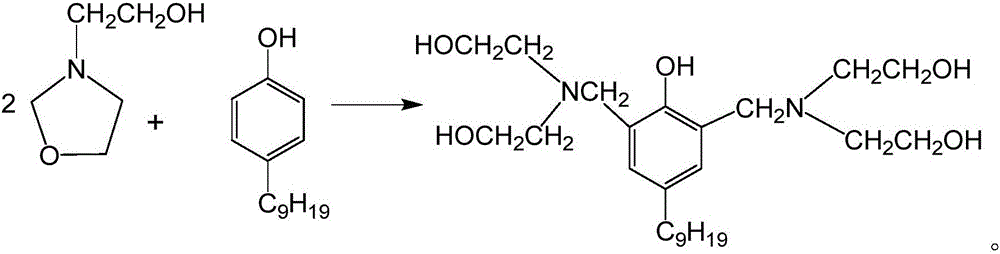
[0048] In the above preparation method, N-hydroxyethyl oxazolidine is formed by dehydration and cyclization reaction of paraformaldehyde and diethanolamine. Its reaction equation is, This reaction is an equilibrium endothermic reaction.
[0049] The temperature of the dehydration cyclization reaction is preferably 50-70° C., and the reaction time is for reference 1.5-2.5 hours, preferably 2 hours. After the reaction is completed, a treatment for separating water may be performed. The separated water is preferably treated until the water content in the...
Embodiment 1
[0061] Put diethanolamine and paraformaldehyde into a three-necked flask, and heat it to about 50 to start the reaction. After 2.5 hours of reaction, dehydration was carried out at 80°C. In the later stage of dehydration, the effect of dehydration can be improved by blowing nitrogen gas. When the tested water content is 0.5%, the dehydration treatment is completed to obtain N-hydroxyethyl oxazolidine, which is ready for use.
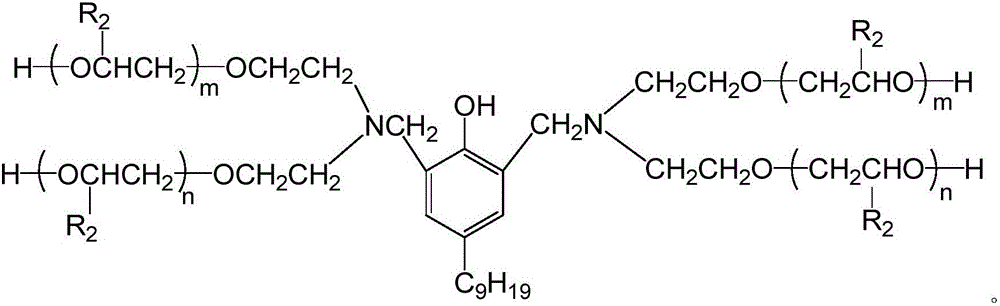
[0062] Transfer nonylphenol and N-hydroxyethyl oxazolidine into the reaction kettle (the relevant parameters of the reaction kettle are: negative pressure is -0.10Mpa, with heating, the maximum temperature is 100°C), and the reaction temperature is controlled to be 80°C , Reaction 3h, to obtain Mannich base.
[0063] Transfer the Mannich base of the above product into an autoclave (the relevant parameters of the reactor are: negative pressure is -0.10 ~ 0.4Mpa, with heating, the maximum temperature is 110°C), vacuumize and replace with nitrogen for 3 t...
Embodiment 2
[0065] Put diethanolamine and paraformaldehyde into a three-necked flask, and heat it to about 70°C to start the reaction. After 1.5 hours of reaction, dehydration was carried out at 100°C. In the later stage of dehydration, the effect of dehydration can be improved by blowing nitrogen gas. When the tested water content is 1%, the dehydration treatment is completed, and N-hydroxyethyl oxazolidine is obtained for future use.
[0066] Transfer nonylphenol and N-hydroxyethyl oxazolidine into the reaction kettle (the relevant parameters of the reaction kettle are: negative pressure is -0.10Mpa, with heating, the maximum temperature is 100°C), and the reaction temperature is controlled at 90°C , Reaction 2h, to obtain Mannich base.
[0067] Transfer the Mannich base of the above product into an autoclave (the relevant parameters of the reactor are: negative pressure is -0.10 ~ 0.4Mpa, with heating, the maximum temperature is 110°C), vacuumize and replace with nitrogen for 3 times...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com