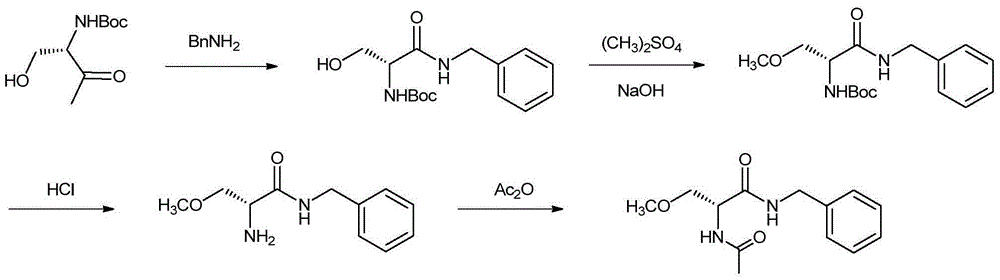
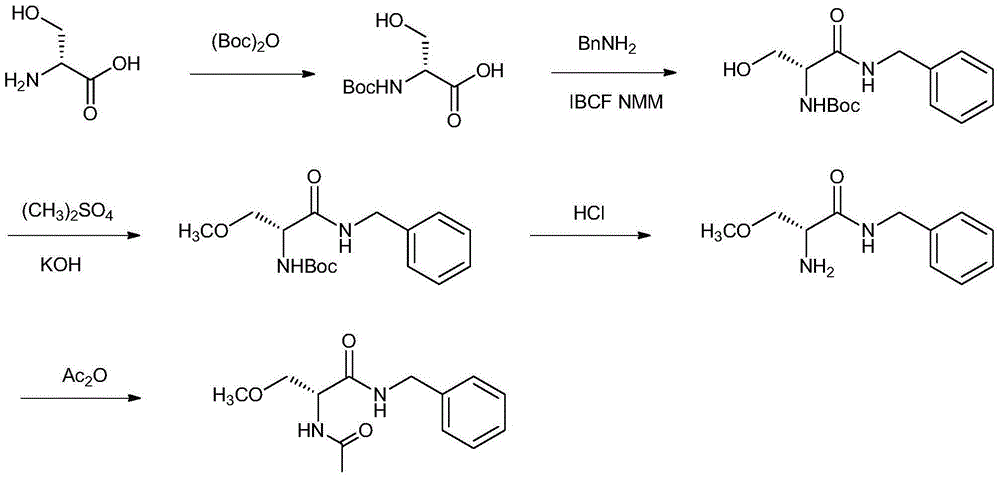
Preparation method for lacosamide
A technology of lacosamide and acetylation, which is applied in the field of preparation of lacosamide, can solve problems such as non-compliance, and achieve the effects of low equipment requirements, high yield, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
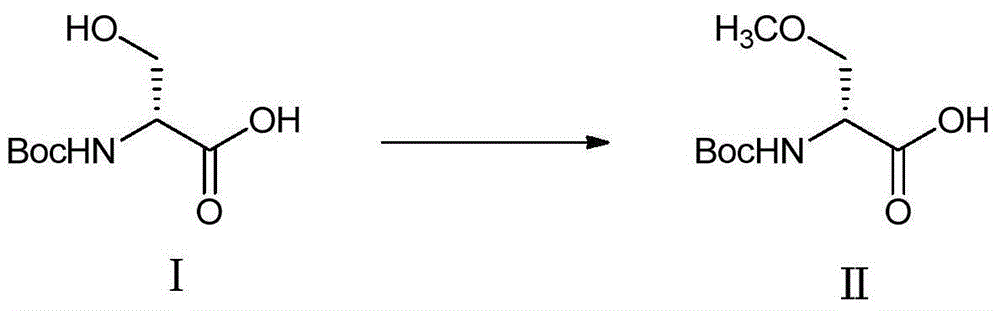
[0036] Example 1 Compound I Generates Compound II
[0037] Into a 1000mL three-necked flask, add 400mL of toluene and 40g of N-tert-butoxycarbonyl-D-serine (Boc-D-serine), lower the stirring temperature to 0-5°C, and add dropwise 36.5g of 30% potassium hydroxide solution. After stirring for 30 minutes, 72.65 g of methyl p-toluenesulfonate and 4 g of tetrabutylammonium bromide were added, and then 43.68 g of 50% potassium hydroxide solution was added dropwise. After reacting at 0-5°C for 8 hours, 200 mL of water was added and the layers were separated. The aqueous layer was washed with 100 mL of toluene, and the organic layer was discarded. The combined aqueous layers were then cooled to below 15°C and acidified with 50% phosphoric acid to pH=2-3.5, extracted with dichloromethane (200 mL*3), and the combined organic layers were added with 30 g of anhydrous sodium sulfate and stirred overnight. The next day it was filtered, and the filtrate was concentrated to dryness under red...
Embodiment 2
[0038] Embodiment 2 compound II generates compound III
[0039] 42.9 g of compound II was dissolved in 400 mL of anhydrous methanol, cooled to 0-5°C, 50 g of thionyl chloride was added dropwise at this temperature, and the solution was raised to room temperature for 2 hours to react. Part of the solvent was distilled off under reduced pressure, and when the remainder was 50 g, 400 mL of dry ethyl acetate was added with stirring. After stirring for 30 minutes, it was filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 32.5 g of a white solid. Yield: 89.2%, HPLC purity: 98.2%. NMR data ( 1 H NMR, DMSO-d6, 400MHz): δppm 8.77 (2H, s, NH), 4.28 (1H, m, CH), 3.79 (2H, d, CH 2 ),3.75(3H,s,CH 3 ),3.29(3H,s,CH 3 ).
Embodiment 3
[0040] Embodiment 3 compound III generates compound IV
[0041] Add 32g of compound III into 300mL of dichloromethane, stir and cool to 0-5°C, add 52g of triethylamine, add 19.8g of acetyl chloride dropwise at 0-5°C, and keep stirring for 4 hours after dropping. Add 150 mL of cold water, separate the layers, take the organic layer, and then wash with 150 mL of 1N hydrochloric acid, 150 mL of water, and 150 mL of saturated sodium chloride solution. Finally, it was dried with 10 g of anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 29.4 g. Yield: 88.2%, HPLC purity: 98.8%, chiral purity: 99.2%. NMR data ( 1 H NMR, DMSO-d6, 400MHz): δppm 8.35 (1H, s, NH), 4.50 (1H, m, CH), 3.64 (3H, s, CH 3 ),3.51(1H,m,CH 2 ),3.34(1H,m,CH 2 ),3.25(3H,s,CH 3 ),1.87(3H,s,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com