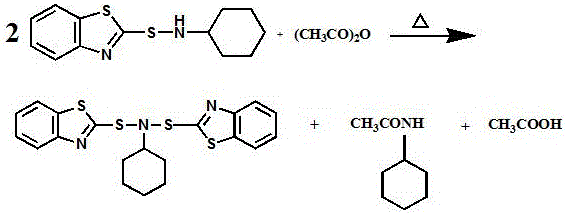
Synthesis method of vulcanization accelerator CBBS (N-cyclohexyl-bis(benzothiazole) sulfonamide)
A technology of benzothiazole sulfenamide and vulcanization accelerator, applied in the direction of organic chemistry, etc., to achieve the effects of long scorch time, high product yield and quality, short reaction time and process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of synthetic method of vulcanization accelerator N-cyclohexyl-bis(benzothiazole)sulfenamide (CBBS), the steps are as follows:
[0028] (1) After nitrogen replacement in the reactor, put 600kg of n-heptane, 600kg of 120# solvent oil and 390kg of acetic anhydride, mix evenly, after heating up to 68-70°C, stir and add 500kg of CBS, add CBS in 4 times, each time 125kg, each interval of 15min, after adding all the CBS, continue to stir at this temperature for 70min to obtain the mixture;
[0029] (2) Add sodium hydroxide solution with a mass concentration of 5-10% into the reaction kettle, adjust the pH value to 7, stir and heat up to 85-95°C, and recover the solvent by distillation. After the solvent distillation is completed, collect it in a storage tank as Organic solvents are recycled. The material in the reaction kettle was filtered, and the obtained filter cake was wet product CBBS. The wet product was dried to obtain 383 kg of off-white solid CBBS. The melting...
Embodiment 2
[0031] According to the method of Example 1 and the synthesis of the vulcanization accelerator N-cyclohexyl-bis(benzothiazole)sulfenamide CBBS, the difference is that the acid anhydride used is maleic anhydride. The obtained CBBS product was 333kg, and its melting point was 118-119°C when tested by a capillary melting point analyzer, its purity was 94% by HPLC, and its yield was 82% based on CBS.
Embodiment 3
[0033] According to the method of Example 1 and the synthesis of the vulcanization accelerator N-cyclohexyl-bis(benzothiazole)sulfenamide CBBS, the difference is that the acid anhydride used is phthalic anhydride. The obtained CBBS product was 325kg, and its melting point was 116-118°C when tested by a capillary melting point analyzer. The purity was 92% by HPLC, and the yield was 80% based on CBS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com