Bismuth/bismuth vanadate composite photocatalyst and preparation method and application thereof to photocatalytic degradation of organics
A technology of bismuth vanadate and compound light, which is applied in the field of photocatalysis, can solve the problem of low quantum yield and achieve high photocatalytic activity, high utilization rate of visible light, and uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of bismuth vanadate precursor: take 1mmol (0.4851g) Bi(NO 3 ) 3 ·5H 2 Dissolve O crystals in 45mL of ethylene glycol solution, stir to make it completely dissolve, and form a transparent solution, which is recorded as A solution; take 1.5mmol (0.1829g) NaVO 3 Dissolve the powder in 27mL of distilled water, stir to dissolve it completely, form a transparent solution, and record it as B solution; add B solution dropwise into A solution to obtain an orange-yellow solution, when B solution is completely added into A solution, record it as B solution. It is solution C; after stirring solution C for ten minutes, transfer it to a 100mL autoclave, place the autoclave in an oven at 180°C, and react for 10h. The synthesized yellow bismuth vanadate sample was washed alternately with ethanol and water, and dried in a vacuum oven at 50°C for 6 hours.
Embodiment 2
[0048] Preparation of bismuth vanadate precursor: take 1mmol (0.4851g) Bi(NO 3 ) 3 ·5H 2 Dissolve O crystals in 45mL of ethylene glycol solution, stir to make it completely dissolve, and form a transparent solution, which is recorded as A solution; take 1.8mmol (0.2195g) NaVO 3 Dissolve the powder in 27mL of distilled water, stir to dissolve it completely, form a transparent solution, and record it as B solution; add B solution dropwise into A solution to obtain an orange-yellow solution, when B solution is completely added into A solution, record it as B solution. It is solution C; after stirring solution C for ten minutes, transfer it to a 100mL autoclave, place the autoclave in an oven at 160°C, and react for 8h. The synthesized yellow bismuth vanadate sample was washed alternately with ethanol and water, and dried in a vacuum oven at 50°C for 6 hours.
Embodiment 3
[0050] Preparation of bismuth vanadate precursor: take 1mmol (0.4851g) Bi(NO 3 ) 3 ·5H 2 O crystals were dissolved in 45mL of ethylene glycol solution, stirred to make it completely dissolved, forming a transparent solution, which was recorded as A solution; take 2.5mmol (0.3035g) NaVO 3 Dissolve the powder in 27mL of distilled water, stir to dissolve it completely, form a transparent solution, and record it as B solution; add B solution dropwise into A solution to obtain an orange-yellow solution, when B solution is completely added into A solution, record it as B solution. It is solution C; after stirring solution C for ten minutes, transfer it to a 100mL autoclave, place the autoclave in an oven at 140°C, and react for 12h. The synthesized yellow bismuth vanadate sample was washed alternately with ethanol and water, and dried in a vacuum oven at 50°C for 6 hours.
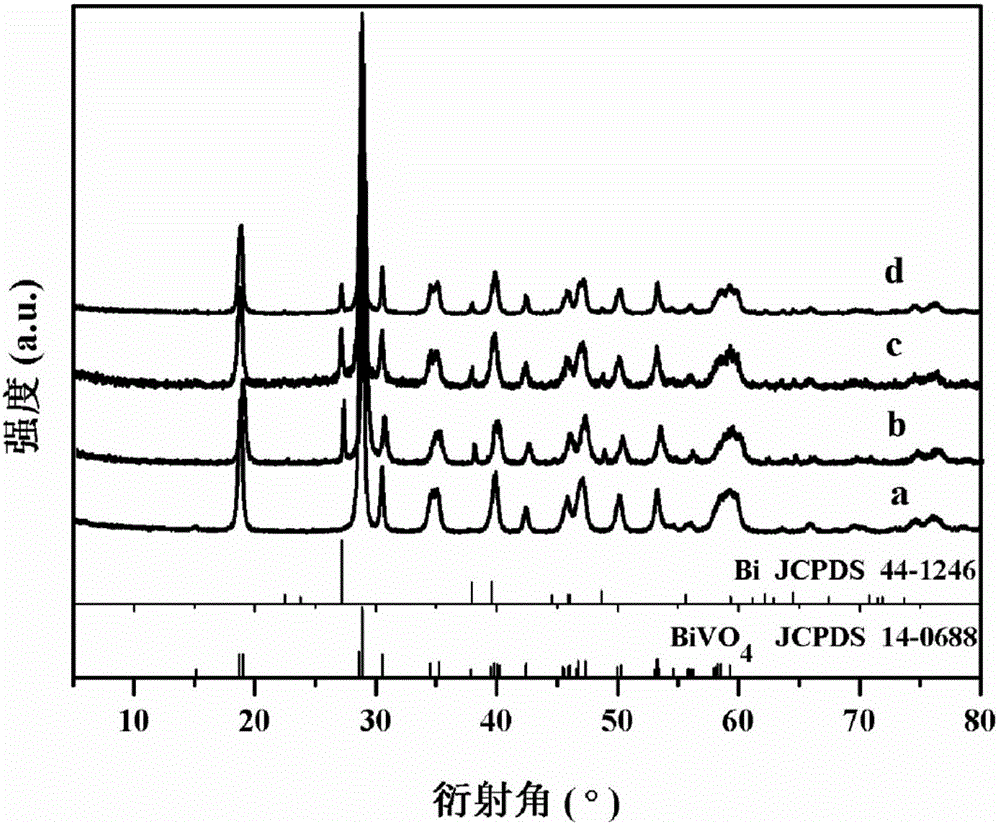
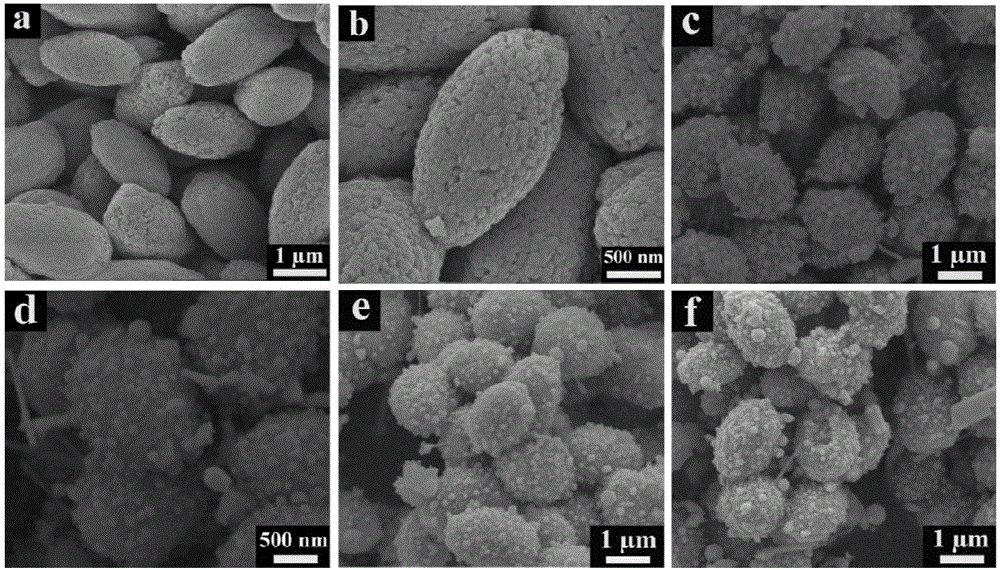
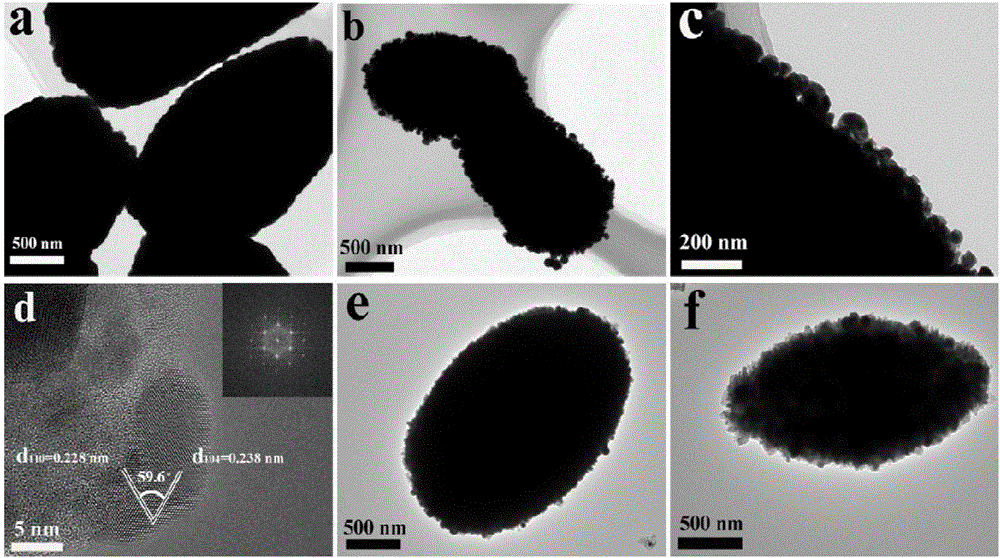
[0051] Some studies on the crystal structure of the bismuth vanadate precursor prepared in Example 1 were c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com