Method for producing thionyl chloride
A technology of thionyl chloride and sulfur, which is applied in the direction of thionyl chloride, sulfur and halogen compounds, etc., can solve the problems of increasing the production load of the rectification tower, reducing the safety performance of the device, and not being able to condense the tail gas, so as to reduce energy consumption and improve equipment and equipment The effect of reducing the load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
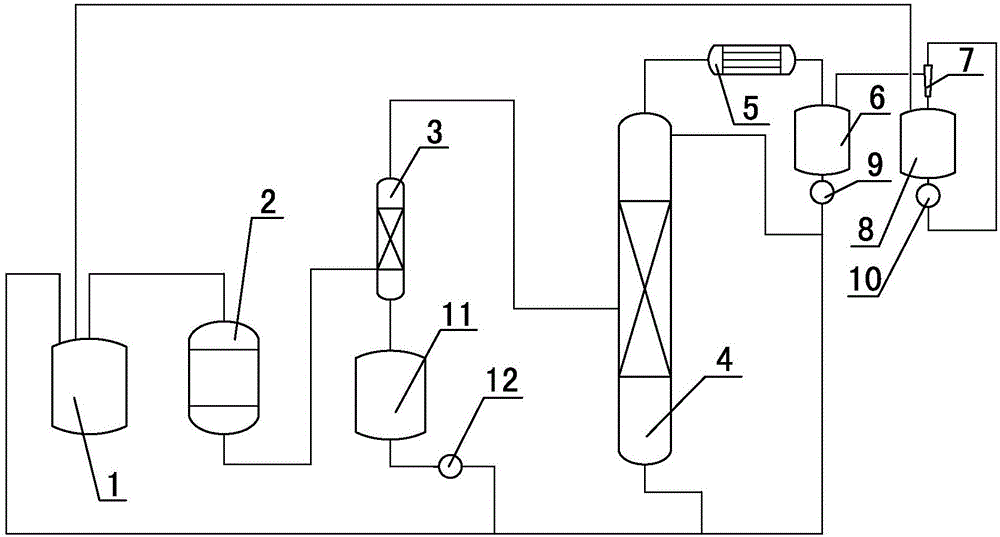
[0020] (1) The synthesis gas produced by the reaction of sulfur and chlorine in the synthesis kettle enters the reactor together with sulfur dioxide gas, and the crude product thionyl chloride generated by the reaction of the reactor directly enters the rectification tower after passing through the absorption tower in gaseous form, and enters the rectification tower in the absorption tower The liquid phase enters the receiving tank;
[0021] (2) The liquid phase in the receiving tank is recycled to the synthesis kettle, and the heavy components produced at the bottom of the rectification tower are recycled to the synthesis kettle. The gas phase at the top of the rectification tower enters the reflux tank after condensation, and part of the liquid phase in the reflux tank passes through the first machine. Pump until the rectification tower is refluxed, and the remaining liquid phase in the reflux tank is collected and used to synthesize the kettle; the volume ratio of part of th...
Embodiment 2
[0024] (1) The synthesis gas produced by the reaction of sulfur and chlorine in the synthesis kettle enters the reactor together with sulfur dioxide gas, and the crude product thionyl chloride generated by the reaction of the reactor directly enters the rectification tower after passing through the absorption tower in gaseous form, and enters the rectification tower in the absorption tower The liquid phase enters the receiving tank;
[0025] (2) The liquid phase in the receiving tank is recycled to the synthesis kettle, and the heavy components produced at the bottom of the rectification tower are recycled to the synthesis kettle. The gas phase at the top of the rectification tower enters the reflux tank after condensation, and part of the liquid phase in the reflux tank passes through the first machine. Pump until the rectification tower is refluxed, and the remaining liquid phase in the reflux tank is taken out and used to synthesize the kettle; the volume ratio of part of th...
Embodiment 3
[0028] (1) The synthesis gas produced by the reaction of sulfur and chlorine in the synthesis kettle enters the reactor together with sulfur dioxide gas, and the crude product thionyl chloride generated by the reaction of the reactor directly enters the rectification tower after passing through the absorption tower in gaseous form, and enters the rectification tower in the absorption tower The liquid phase enters the receiving tank;
[0029] (2) The liquid phase in the receiving tank is recycled to the synthesis kettle, and the heavy components produced at the bottom of the rectification tower are recycled to the synthesis kettle. The gas phase at the top of the rectification tower enters the reflux tank after condensation, and part of the liquid phase in the reflux tank passes through the first machine. The pump is pumped until the rectification tower is refluxed, and the remaining liquid phase in the reflux tank is taken out and used to synthesize the kettle; the volume ratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com