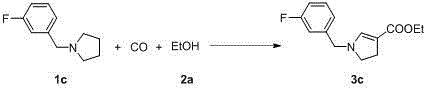
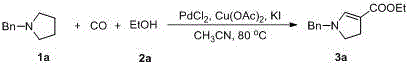Synthetic method for pyrroline-3-formic ether compounds
A technology of ester compounds and dihydropyrrole, which is applied in the field of synthesis of dihydropyrrole-3-carboxylate compounds, can solve the problems of difficult availability of raw materials, atom economy, harsh reaction conditions, expensive catalysts, etc., to avoid intermediates Separation and purification process, easy operation, and the effect of reducing environmental burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016] Add 1a (0.5 mmol, 81 mg) and acetonitrile (CH 3 CN, 5 mL), then 2a (5 mmol, 292 µL), palladium chloride (PdCl 2 , 0.05 mmol, 9 mg), copper acetate (Cu(OAc) 2 , 0.5 mmol, 91mg) and potassium iodide (KI, 0.5 mmol, 83 mg). In a CO / air (1 atm) atmosphere, stir the reaction at 80°C for 12 hours, then add 10 mL of saturated sodium chloride solution to quench the reaction, extract with ethyl acetate (10 mL × 3), combine the organic phases, and use water and sodium sulfate to dry. After filtration, spin-drying, and separation on a silica gel column (petroleum ether / ethyl acetate = 20:1), the yellow liquid product 3a (79 mg, 68%) was obtained. The characterization data of this compound are as follows: 1 H NMR (400 MHz, CDCl 3 ) δ 1.26 (t, J = 7.2Hz, 3H), 2.79 (t, J = 10.0 Hz, 2H), 3.37 (t, J = 10.4 Hz, 2H), 4.14 (q, J =7.2 Hz, 2H), 4.23 (s, 2H), 7.17 (s, 1H), 7.25-7.37 (m, 5H). 13 C NMR (100 MHz, CDCl 3 ) δ 14.7, 27.7, 54.0, 54.6, 58.9, 101.4, 127.1, 1...
Embodiment 2
[0018] According to the method described in Example 1, 1a (0.5 mmol, 81 mg) and acetonitrile (5 mL) were added to a 25 mL reaction vial, and then 2a (5 mmol, 292 µL), palladium acetate (0.05 mmol, 11 mg ) and copper acetate (0.5 mmol, 91mg). In a CO (1 atm) atmosphere, stir the reaction at 80°C for 12 hours, then add 10 mL of saturated sodium chloride solution to quench the reaction, extract with ethyl acetate (10 mL × 3), combine the organic phases, and wash with anhydrous sulfuric acid Sodium dry. After filtration, spin-drying, and silica gel column separation (petroleum ether / ethyl acetate = 20:1), the yellow liquid product 3a (47 mg, 41%) was obtained.
Embodiment 3
[0020] According to the method described in Example 1, 1a (0.5 mmol, 81 mg) and acetonitrile (5 mL) were added to a 25 mL reaction vial, and then 2a (5 mmol, 292 µL), tris(dibenzylideneacetone) Dipalladium (0.05 mmol, 46 mg) and copper acetate (0.5 mmol, 91 mg). In a CO (1 atm) atmosphere, stir the reaction at 80°C for 12 hours, then add 10 mL of saturated sodium chloride solution to quench the reaction, extract with ethyl acetate (10 mL × 3), combine the organic phases, and wash with anhydrous sulfuric acid Sodium dry. After filtration, spin-drying, and silica gel column separation (petroleum ether / ethyl acetate = 20:1), the yellow liquid product 3a (32 mg, 28%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com