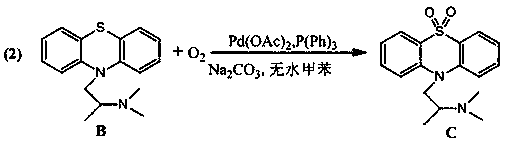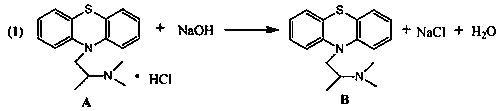A kind of preparation method that uses oxygen as oxidant to prepare dioxpromazine hydrochloride
A technology of dioxpromazine hydrochloride and oxidant, applied in the direction of organic chemistry, etc., can solve the problems of increased difficulty in processing work, poor production safety, complicated production process, etc., and achieve the effects of reduced production cost, low cost, and improved experimental safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the synthesis of intermediate B
[0016]
[0017] In a 250ml three-neck flask, add 100ml of weighed pure water and 2.0g of promethazine hydrochloride A in sequence, and stir on a magnetic stirrer for 10min until all the materials are dissolved. After complete dissolution, slowly add the prepared lye ((600g sodium hydroxide + 1400g drinking water)) to adjust the pH to 12, the temperature is controlled at 25°C, the liquid dropping rate is 60 drops per minute, and the stirring is continued until the pH is stable. Add 20ml of dichloromethane to extract the promethazine in the reaction solution, repeat the washing and extraction operation 3 times, after drying over anhydrous magnesium sulfate, extract the solvent, and the thick product is purified by column chromatography to obtain 1.69g of white solid promethazine. Process yield: 95.1%. MS(EI):m / z:284.1349([M] + ).
Embodiment 2
[0018] Embodiment 2: the synthesis of intermediate C
[0019]
[0020] Take by weighing substrate 1.78g promethazine, 0.28g palladium acetate, 2.65g sodium carbonate, in dry 250ml two-necked flask, add 100ml anhydrous toluene as reaction solvent, feed oxygen (flow velocity is 8m / s, tube diameter area 9×10 -6 m 2 ), be heated to 80 ℃ of reaction 18 hours; Described substrate promethazine: triphenylphosphine: the mol ratio of anhydrous sodium carbonate is 1:0.5:4, and the consumption of described palladium acetate is that of substrate promethazine 2mol%, after the reaction is over, after the solvent is extracted under reduced pressure, the reaction solution is extracted three times with dichloromethane, the extracts are combined and dried with anhydrous magnesium sulfate, the solvent is spin-dried, and dried; the crude product is purified by column chromatography to obtain the target product C1 .78g, the yield of this process was 90.2. MS(EI):m / z:316.1245([M] + ).
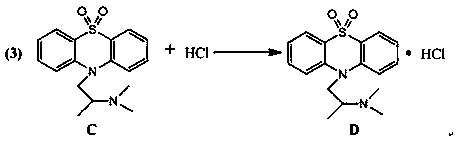
Embodiment 3
[0021] Embodiment 3: the synthesis of target product dioxetazine hydrochloride D
[0022]
[0023] In a 500ml three-necked flask, add 1.78g of promethazine, add acetone as a solvent, and stir on a magnetic stirrer until all the materials are dissolved. After feeding, the temperature was lowered to 20°C. (keep the liquid temperature at 20°C, slowly pass 300g of hydrochloric acid gas into 500ml of acetone for use), slowly add the pre-prepared acetone hydrogen chloride solution dropwise (keep the liquid temperature at 20°C, slowly pass 300g of hydrochloric acid gas ( Pass through for about 1 hour) into 900g of acetone for later use) to pH = 4, stir until the pH is stable, and obtain an off-white suspension of dioxpromazine hydrochloride acetone solution, add activated carbon, stir for 35 minutes to decolorize, after the decolorization is completed, filter In addition to activated carbon, the activated carbon filter cake was rinsed with 200ml acetone, and the washing liquid wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com