Preparation method and application of virus Ankara subunit vaccine
An Ankara virus and subunit vaccine technology, applied in the field of Ankara virus subunit vaccine preparation, can solve problems such as toxic organisms, adverse immune responses, economic losses and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
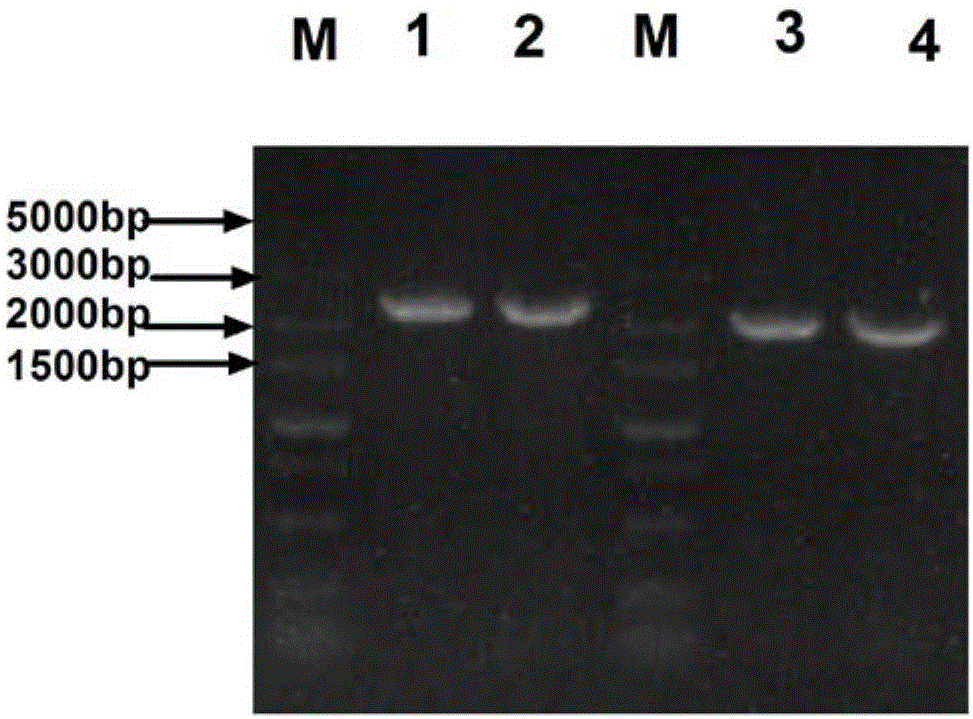
[0105] Embodiment 1: Acquisition of recombinant baculovirus Ac-Penton
[0106]1. Acquisition of Penton gene
[0107] Extraction of Ankara virus DNA:
[0108] Viral DNA was extracted using the SDS-Protease K-phenol / chloroform method, that is, 600 μL of the virus liquid was centrifuged at 4°C and 12,000 rpm / min for 10 minutes, and 425 μL of the supernatant was added to 20 μL of 25 mg / mL proteinase K and 50 μL of 10% SDS to make it Proteinase K and SDS final concentrations were 1 mg / ml and 1%. Water bath at 56°C for 1 hour and shake occasionally; add an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1) and mix thoroughly, centrifuge at 10,000 rpm at 4°C for 15 minutes. Take the supernatant, add an equal volume of chloroform to the supernatant and mix thoroughly at 4°C at 10,000 rpm, centrifuge for 15 minutes, take the supernatant, add 1 / 10 volume of NaAc (3M, pH 5.0) and more than 2 times the volume of absolute ethanol and mix well. Precipitate at -20°C for 2 hours, ...
Embodiment 2
[0148] Embodiment 2: Expression, quantification and purification of recombinant protein Penton
[0149] 1. Western Blotting to detect the expression of the target protein Penton
[0150] Cells infected with P3 recombinant baculovirus and normal cells were lysed with cell lysate on ice for 30 minutes, added to 5×SDS Loading Buffer, boiled in boiling water for 5-8 minutes, and then separated, followed by conventional methods for 10 minutes. SDS-PAGE electrophoresis analysis of % separating gel and 5% stacking gel, transfer to PVDF membrane with 5% skimmed milk powder, block overnight at 4°C, add 1:5000 diluted His tag primary antibody, shake at room temperature for 2-3h, TBST After washing 3 times, add AP-labeled rabbit anti-mouse secondary antibody diluted 1:5000, shake at room temperature for 1 hour, wash 3 times with TBST, and use NBT / BCIP system to develop color. Observation of specific protein bands indicated that Penton protein had been expressed. (Such as Figure 6 sho...
Embodiment 3
[0159] Example 3: Composition, preparation and detection of Ankara subunit vaccine oil emulsion
[0160] Antigen: 500μg / ml purified target protein Penton
[0161] Adjuvant: ISA 71 VG water-in-oil (W / O)
[0162] Mass ratio of antigen to adjuvant: 3:7
[0163] Vaccine preparation: Dilute the purified target protein Penton to 500 μg / ml and add it to the container, then drop the adjuvant ISA 71 VG into the container according to the above ratio for emulsification.
[0164] Vaccine property test: after emulsification, the vaccine is dripped into clear water, the first drop will disperse, and the second drop will be in the form of oil beads, and the oil beads on the liquid surface will not break the emulsification.
[0165] Sterility test: Take a small amount of the vaccine and coat it on a TSA plate, and incubate in a 37°C incubator for 18 hours without colonies growing.
[0166] Stability test: Centrifuge at 3000rpm / min for 15 minutes, the emulsified vaccine is not separated af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com