Treatment method for gold nanoparticles protected by organic functional group
A technology of gold nanoparticles and organic functional groups, which is applied in the field of nanomaterials, can solve the problems of inapplicable excision, etc., and achieve the effects of improved catalytic activity, wide selection range, and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Au 25 (SR) 18 Synthesis
[0028] Weigh 60 mg of HAuCl 4 4H 2 O was added to a 50mL three-necked flask, and 15mL THF was added, and the solution was golden yellow. Add TOAB 92 mg (TOAB: Au = 1.16 mol: 1 mol), and the solution gradually turns from golden yellow to orange red. After stirring for 30min, add PhCH 2 CH 2 SH 97μL (PhCH 2 CH 2 SH: Au=5mol:1mol), when the color of the solution gradually becomes lighter to colorless, add NaBH 4 (4mL ice water) 55mg (NaBH 4 : Au=10mol:1mol). Stir for three hours to stop the reaction. Wash the product with n-hexane and methanol, dissolve the precipitate with dichloromethane, remove insoluble matter, and obtain Au after drying 25 (SR) 18 .
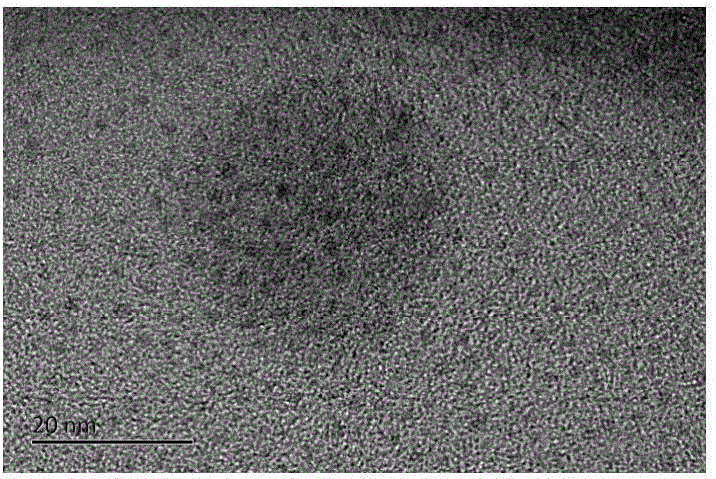
[0029] Such as figure 1 It is a transmission electron microscope picture of the gold nanocluster prepared in Example 1, and the diameter of the gold particle is about 1.5 nm.
Embodiment 2
[0030] Example 2: Au 25 (SR) 18 Ammonia
[0031] At room temperature, add equal volumes of dichloromethane and methanol into the sample vial to dissolve 1mg of Au in 1mL 25 (SR) 18 , and then add 5 μL of ammonia water (NH 3 ·H 2 O, mass percent concentration is 25%), obtains the sample before processing.
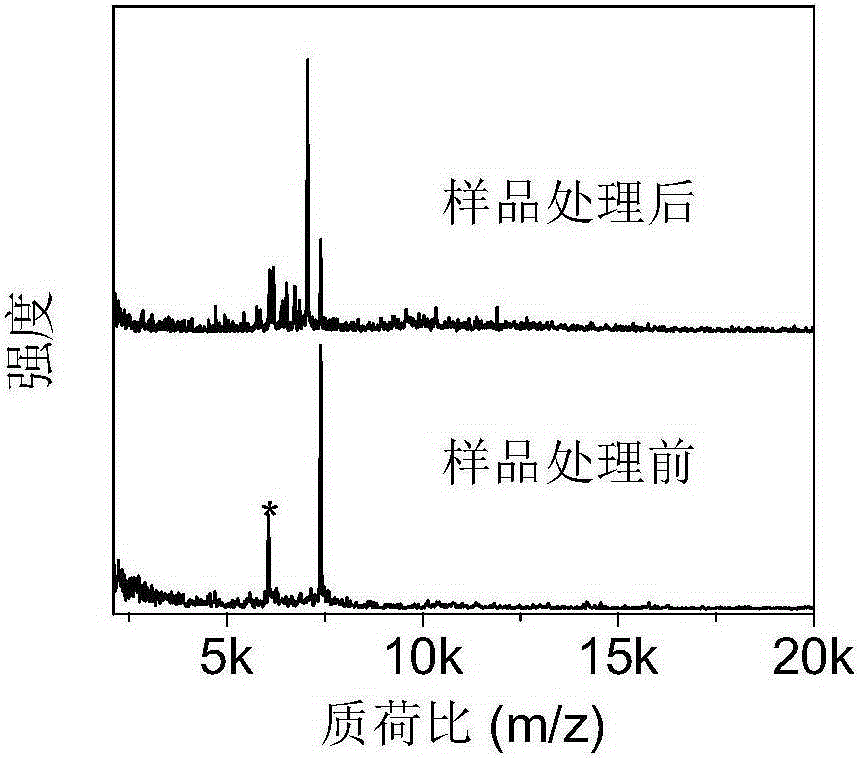
[0032] Such as figure 2 The spectrogram below is the Au that only adds ammoniacal liquor in embodiment 2 25 (SR) 18 mass spectrum. It can be seen from the figure that it corresponds to Au 25 (SR) 18 The mass-to-core ratio (m / z: 7394.3) and its fragment mass-to-core ratio (m / z: 6058.1).
Embodiment 3
[0033] Embodiment 3: sample uses Co(OAc) 2 deal with
[0034] Add 1mL equal volumes of dichloromethane and methanol into the vial to dissolve 1mg of Au 25 (SR) 18 , add 5 μL ammonia water (NH 3 ·H 2 O, the mass percent concentration is 25%), and then add 0.14 μmol of Co(OAc) 2 (Co 2+ : Au 25 (SR) 18 =2:1), stirred at room temperature for 10 h.
[0035] Such as figure 2The above spectrogram in the middle is that sample uses Co(OAc) in embodiment 3 2 The processed mass spectrum. It can be seen from the figure that it corresponds to Au 24 (SR) 17 The mass-to-core ratio is 7060.3. This peak does not appear in the figure below, proving that Au 24 (SR) 17 It did not appear in the mass spectrometry test, but Co(OAc) 2 processing results.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com