A kind of preparation method of fullerene polyaza bridge water-soluble derivative
A technology of fullerene polyaza bridges and derivatives, which is applied in the field of carbon nanomaterials, can solve the problems of increasing the difficulty of mass production operations, low dissolution of fullerenes, and inability to achieve large-scale preparation of fullerene products. The effect of excellent solubility in water phase, simple operation method and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
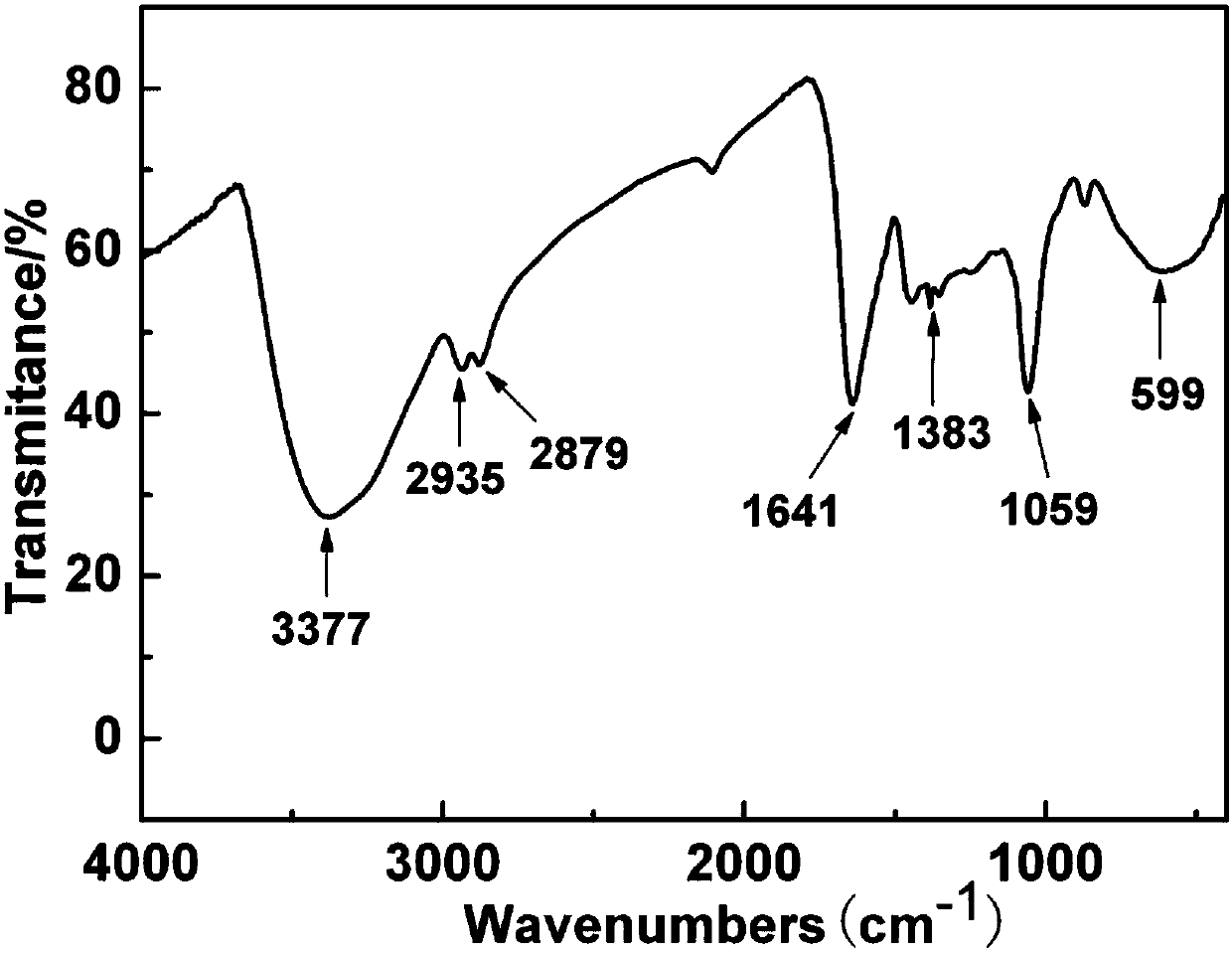
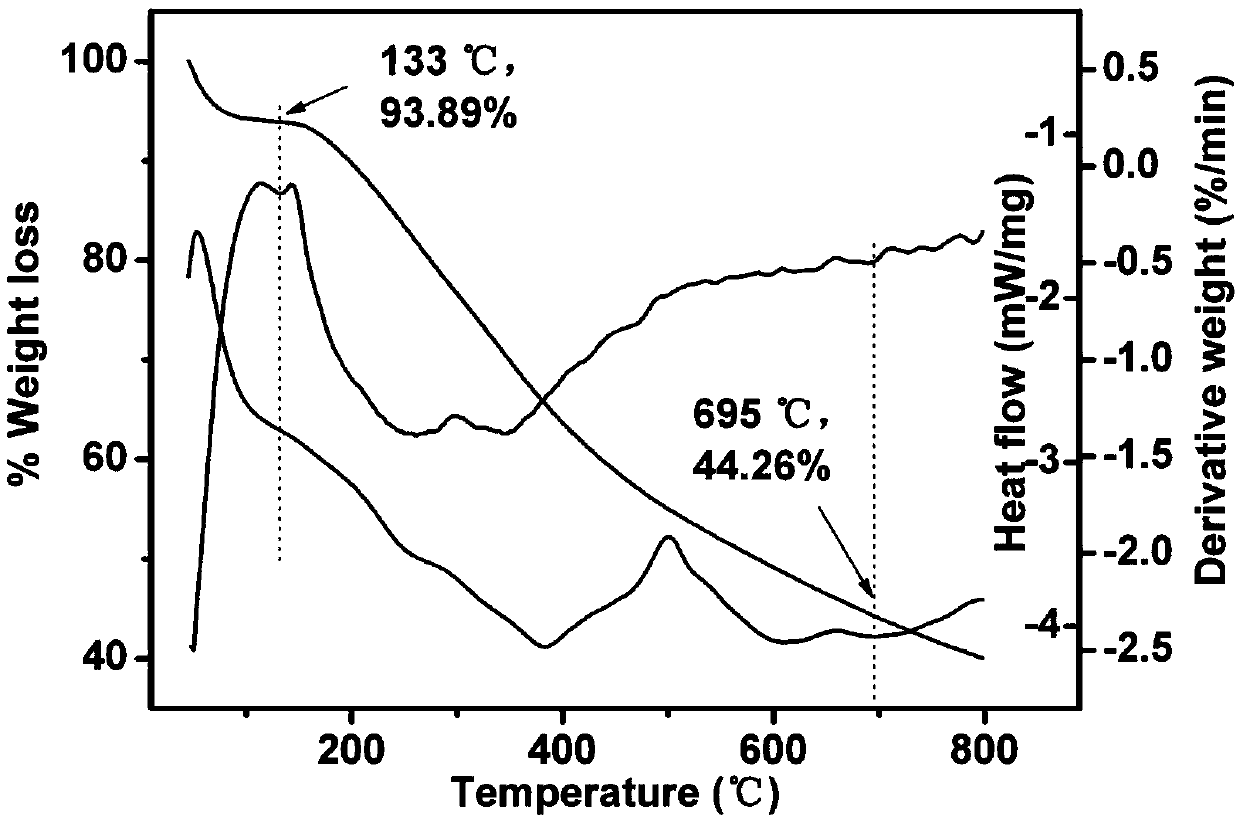
[0043] Example 1: Large scale preparation of C 60 Water-soluble product C with azide ethanol 60 (NCH 2 CH 2 Oh) 14 (Azafullerol 1)
[0044] (1) Add 60.0g C 60 Solid powder and 362.8g of liquid 2-azidoethanol (substance ratio: 1:50) were respectively fed into a 500mL three-necked reaction bottle, connected to a condenser tube and a mechanical stirring device, without adding an organic solvent, directly in an inert gas atmosphere Under mechanical stirring;
[0045] (2) The reaction system is warmed up to reflux, and after being incubated for 72 hours, the heating is stopped;
[0046] (3) Part of the unreacted 2-azidoethanol is removed by distillation under reduced pressure, and the residue is washed repeatedly with ether and ethyl acetate to further remove unreacted 2-azidoethanol and non-water-soluble alcohols with a small number of additions. Derivatized product, then add deionized water and ultrasonically extract for several times until the supernatant is colorless, th...
Embodiment 2
[0054] Example 2: Preparation C 60 Water-soluble addition product C with azidoethylamine 60 (NCH 2 CH 2 NH 2 ) 12 (Azafulleramine 1)
[0055] (1) 12.0g C 60 The solid powder and 143.5g of liquid 2-azidoethylamine (the amount of material to feed ratio is 1:100) are respectively fed into a 250mL three-port reactor, connected to a condenser tube and a mechanical stirring device, without adding an organic solvent, directly in an inert gas Mechanical stirring under atmosphere;
[0056] (2) The reaction system is warming up to reflux, and after being incubated for 48 hours, the heating is stopped;
[0057] (3) Part of the unreacted 2-azidoethylamine is removed by distillation under reduced pressure, and the residue is washed repeatedly with ether and ethyl acetate to further remove unreacted 2-azidoethylamine and the lesser addition number. The non-water-soluble derivatized product was then ultrasonically extracted with deionized water for several times until the supernatant w...
Embodiment 3
[0059] Example 3: Preparation C 60 A water-soluble derivative of fullerene-based polyglycine with azidoacetic acid C 60 (NCH 2 COOH) 13
[0060] (1) Add 20.0g C 60 The solid powder and 140.4g of liquid 2-azidoacetic acid (the amount of material to feed ratio is 1:100) were respectively fed into a 250mL three-port reactor, connected to a condenser tube and a mechanical stirring device, without adding an organic solvent, directly in an inert gas atmosphere Under mechanical stirring;
[0061] (2) The reaction system is heated to reflux, and after being incubated for 12 hours, the heating is stopped;
[0062] (3) Part of the unreacted 2-azidoacetic acid is removed by distillation under reduced pressure, and the residue is washed repeatedly with ether and ethyl acetate to further remove unreacted 2-azidoacetic acid and non-water-soluble compounds with a small number of additions. The derivatized product was then ultrasonically extracted with deionized water for several times ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com