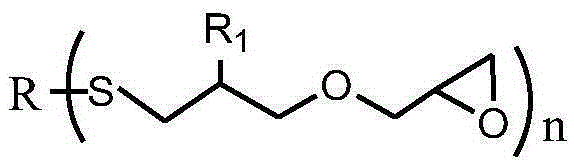
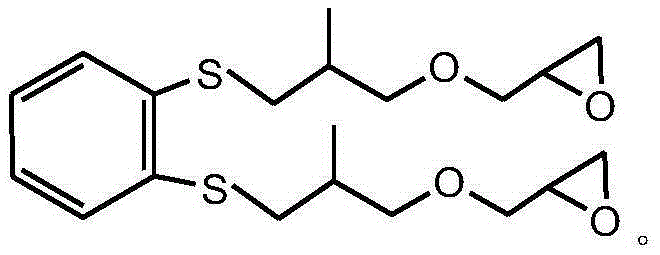
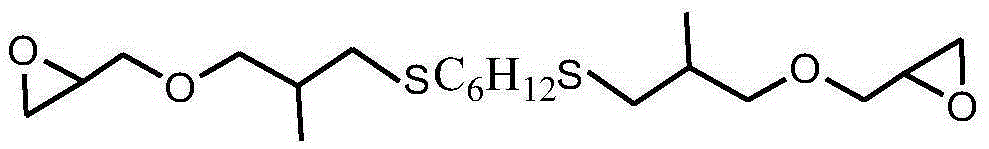Sulfur-containing glycidyl ether epoxy resin and preparation method thereof
A technology of glycidyl ether and epoxy resin, which is applied in the field of sulfur-containing glycidyl ether epoxy resin and its preparation, can solve the problems of long reaction time, low viscosity, and high product viscosity, and achieve high storage stability and excellent preparation process. Simple, low reaction temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] After mixing 0.1mol (11.0g) thiophenol, 0.101mol (11.3g) allyl glycidyl ether, 5.5g methanol and 0.06g benzophenone photoinitiator, use 500W ultraviolet light at room temperature React for 10 minutes, vacuum out the organic solvent and excess allyl glycidyl ether to obtain a sulfur-containing glycidyl ether epoxy resin. The molecular weight of the test is 222g / mol, the viscosity at 25°C is 620cp, and the epoxy value is 0.45mol / mol. 100g, the yield is 99.0%, and the viscosity of the product only increases by 0.9% under the storage condition of 60°C / 96h.
[0037] The sulfur-containing glycidyl ether epoxy resin structural formula prepared by the present embodiment is as follows:
Embodiment 2
[0039] After mixing 0.1mol (9.0g) mercaptobutane, 0.14mol (15.7g) allyl glycidyl ether, 27.0g methanol and 0.27g benzophenone photoinitiator, use 1000W ultraviolet light at room temperature React for 5 minutes, vacuum out the organic solvent and excess allyl glycidyl ether to obtain a sulfur-containing glycidyl ether epoxy resin. The molecular weight is 202g / mol, the viscosity at 25°C is 450cp, and the epoxy value is 0.50mol / mol. 100g, the yield is 99.5%, and the viscosity of the product only increases by 1.0% under the storage condition of 60°C / 96h.
[0040] The sulfur-containing glycidyl ether epoxy resin structural formula prepared by the present embodiment is as follows:
Embodiment 3
[0042] After mixing 0.1mol (17.4g) mercapto n-decane, 0.2mol (25.2g) 2-methallyl glycidyl ether, 73.8g tetrahydrofuran and 0.49g p-aminopropiophenone photoinitiator, under room temperature React with 1500W ultraviolet light for 3 minutes, vacuum out the organic solvent and excess 2-methallyl glycidyl ether, to obtain sulfur-containing glycidyl ether epoxy resin, the molecular weight of the test is 302g / mol, and the viscosity at 25°C is 600cp, the epoxy value is 0.27mol / 100g, the yield is 98.6%, and the viscosity of the product only increases by 1.1% under the storage condition of 60°C / 96h.
[0043] The sulfur-containing glycidyl ether epoxy resin structural formula prepared by the present embodiment is as follows:
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com