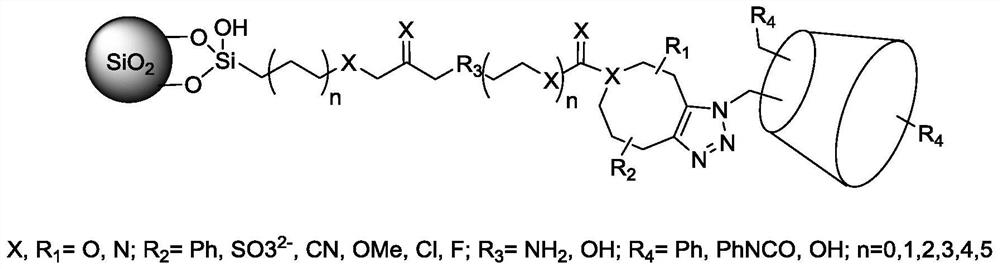
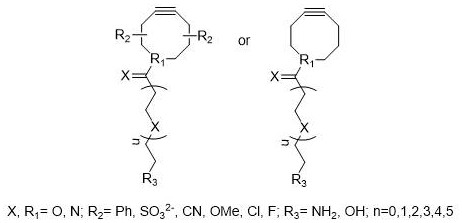
A kind of preparation method of cyclodextrin chromatography stationary phase
A chromatographic stationary phase and cyclodextrin technology, applied in the field of cyclodextrin-modified silica gel stationary phases, can solve the problems of difficult removal, restricting stationary phase separation efficiency, etc., and achieve the effects of high selectivity, saving reaction time, and avoiding residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Under nitrogen protection, take 1.13g of epoxypropyltrimethoxysilane reagent, dissolve it in 20mL of anhydrous tetrahydrofuran solution, stir it electromagnetically, add 431mg of ADIBO reagent containing amino groups at the end and phenyl groups on both sides, and adjust with triethylamine. pH value, stirred at room temperature, and detected by liquid chromatography to obtain the ADIBO-modified silane reagent. Under nitrogen protection, dissolve the dried silica gel (1.5g) in 30mL of anhydrous toluene, add the ADIBO silane reagent prepared above, and dropwise add pyridine (6mmol, 480μL), heat to reflux, react for 24h, stop the reaction, and cool Suction filtration, washing with anhydrous toluene, tetrahydrofuran, methanol, water, methanol successively, drying and solidification at 80°C for 6 hours, to obtain silica gel activated by ADIBO reagent, named as S-oxy-ADIBO material.
[0024] Weigh 1.0 g of the above dried S-oxy-ADIBO material, under nitrogen protection, disso...
Embodiment 2
[0026] The difference from Example 1 is that the alkoxysilane reagent is a triethoxyisocyanosilane reagent, which (2.47g, 10mmol) is dissolved in 20mL of anhydrous THF solution, stirred electromagnetically, and added with an amino group at the end , ADIBO reagent (4.22 g, 10 mmol) containing methoxyphenyl groups on both sides, stirred at room temperature, and detected by liquid chromatography to obtain ADIBO modified isocyanosilane reagent. Under nitrogen protection, dissolve the dried silica gel (2.5g) in 30mL of anhydrous toluene, add the ADIBO silane reagent prepared above, and dropwise add pyridine (6mmol, 480μL), heat to reflux, react for 24h, stop the reaction, and cool Suction filtration, followed by washing with anhydrous toluene, tetrahydrofuran, methanol, water, methanol, drying and solidification at 80°C for 6 hours, to obtain silica gel activated by ADIBO reagent, named S-isocyano-MeO-ADIBO material;
[0027]Weigh 1.5g of the above dried S-isocyano-ADIBO material, ...
Embodiment 3
[0029] The difference from Example 2 is that the alkoxysilane reagent is an aminopropyltrimethoxysilane reagent modified by glutaraldehyde, and the ADIBO reagent has an amino group at the end and a phenyl group substituted by a sulfonic acid group on both sides; the cyclodextrin reagent First carried out derivatization, the derivatization reagent is isocyanobenzene, and the specific implementation method is:
[0030] At room temperature, to the prepared DMF solution (30mL) of cyclodextrin azide (3.48g, 3mmol), slowly add isocyanobenzene reagent (12mL, 60mmol) dropwise, add pyridine (30mL) and stir for 12h, pump Filter, and recrystallize the obtained isocyanobenzene derivatized azidocyclodextrin CPCD with acetone;
[0031] Dissolve glutaraldehyde-modified aminopropyltrimethoxysilane reagent (2.0g, 5mmol) in 20mL of anhydrous THF solution, stir electromagnetically, add ADIBO with amino groups at the end and phenyl groups substituted with sulfonic acid groups on both sides. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com