Sulfated hyaluronic acid 2-octyldodecyl ester
A technology of octyldodecyl ester and hyaluronic acid, which is applied in the direction of microcapsules, capsule delivery, nanocapsules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 hyaluronic acid tetrabutylammonium salt
[0029] Sodium hyaluronate (MW5000, HA-Na, 2.0g) was dissolved in 400mL double-distilled water, and magnetically stirred overnight at room temperature; another 20g DowexWX8 cation exchange resin (tetrabutylammonium type) was weighed and added to HA-Na, and magnetically stirred at room temperature Overnight, filter, lyophilize and vacuum-dry at 40°C to obtain hyaluronic acid tetrabutylammonium salt (HA-TeBA).
Embodiment 2
[0030] The preparation of embodiment 2 sulfated hyaluronic acid (sHA)
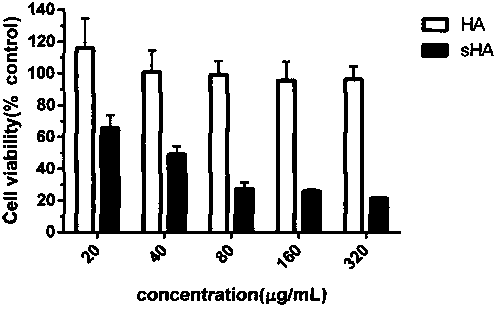
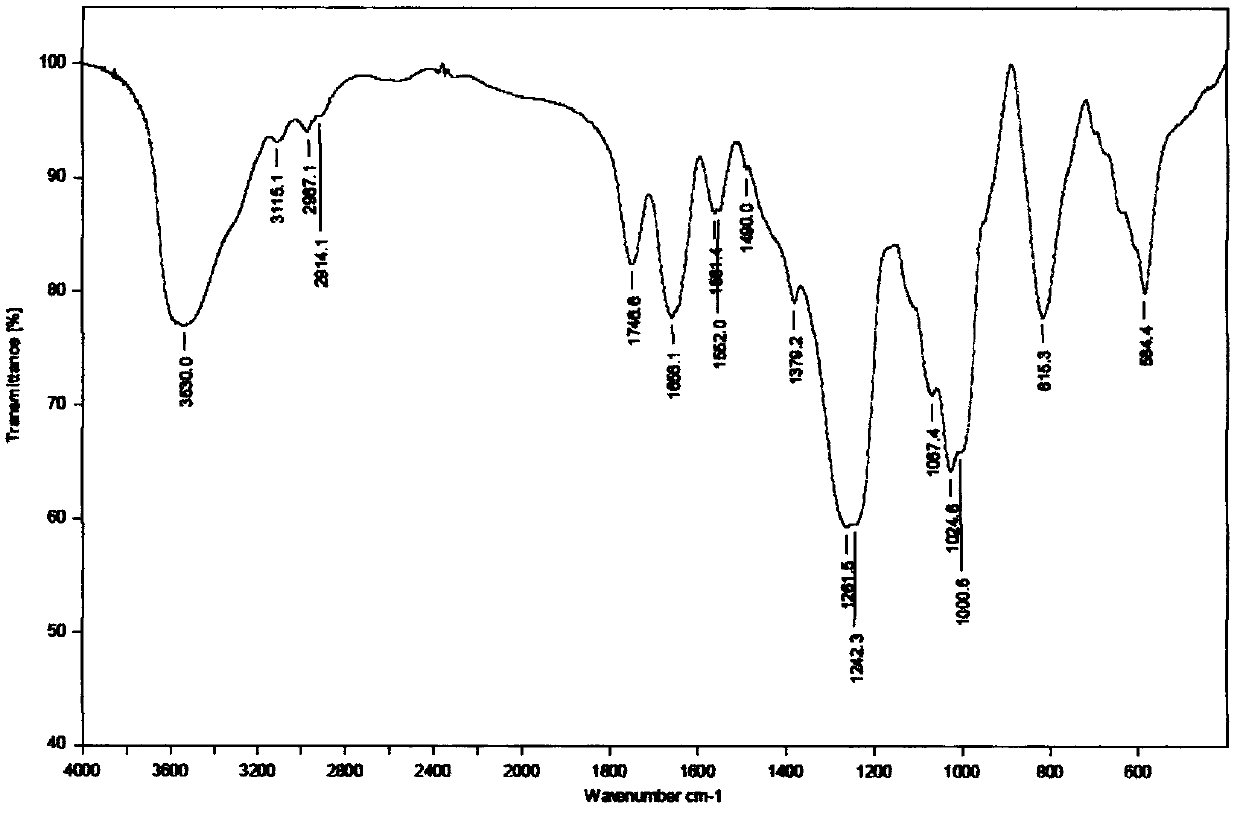
[0031] Accurately weigh the HA-TeBA (MW5000, 1000mg) in Example 1 and dissolve it in 50ml of N,N'-dimethylformamide (DMF), stir magnetically at 50°C for 2h to dissolve it; in addition, accurately weigh 1540mg of sulfated Sulfur trioxide DMF complex (SO 3DMF) was dissolved in 50mL DMF, stirred at room temperature until dissolved, then added to HA-TeBA, protected with nitrogen, stirred magnetically for 1h after ice bathing; adjusted pH to neutral with 1M NaOH solution, added ethanol for precipitation, centrifuged, and dialyzed with distilled water for 2d , freeze-dried in vacuum to obtain sulfated hyaluronic acid (sHA). The IR spectrum confirmed the structure of sHA (see figure 1 ). The degree of sulfation was determined by elemental analysis, and the sulfur content (S%) was 4.392%.
Embodiment 3
[0032] Example 3 Preparation of Sulfated Hyaluronic Acid 2-Octyldodecanyl Ester (sHA-OCD)
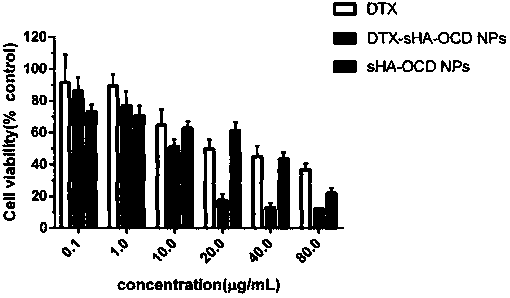
[0033] Accurately weigh the sHA (100 mg) in Example 2 and dissolve it in 5 ml of formamide, stir magnetically at room temperature for 20 minutes to dissolve, then ice-bath, add 1-ethyl-(3-dimethylaminopropyl)carbodiethylene Amine hydrochloride (EDC, 95mg), N-hydroxysuccinimide (NHS, 58mg), react in ice bath for 2h; another precise amount of 2-octyldodecanol (OCD) was dissolved in 9mL of DMF , was added dropwise to the sHA solution, and stirred magnetically at 35°C for 24 hours; the reaction solution was precipitated with ethanol, centrifuged, dissolved in water, dialyzed in distilled water for 2 days, and freeze-dried in a vacuum to obtain sulfated hyaluronic acid 2-octyldodecyl ester ( sHA-OCD). 1 H-NMR spectrum (see figure 2 ) confirmed the sHA-OCD structure, and the substitution degree was about 10%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com