Kit for rapidly detecting respiratory viruses and preparation method of kit
A technology of respiratory tract and detection test strips, which is applied in the field of medical diagnosis, can solve the problems of unsuitable on-site instant detection, unsuitable clinical screening, and long time consumption, so as to reduce the risk of crowd infection, save disposable consumables, and shorten the detection cycle. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
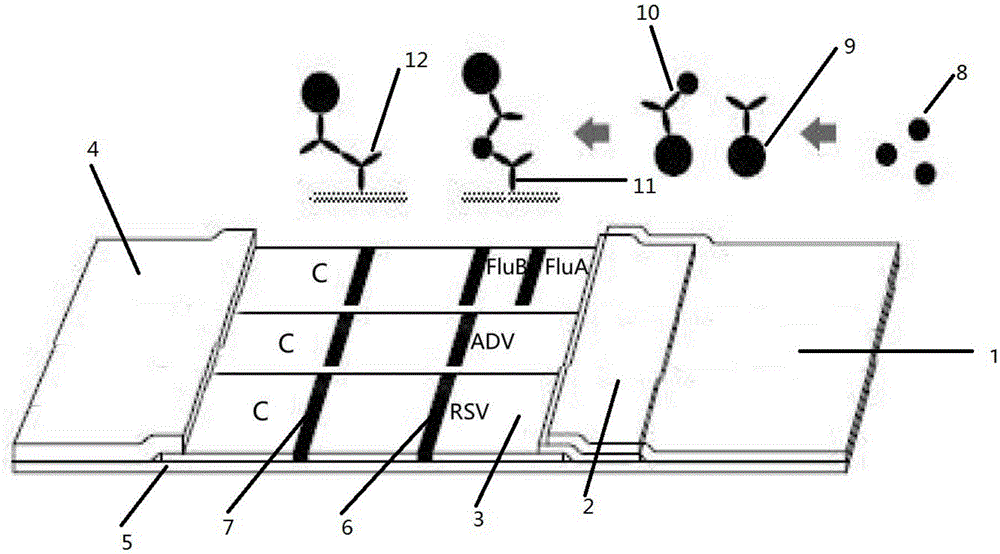
[0038] The preparation of embodiment 1 respiratory virus rapid detection test paper
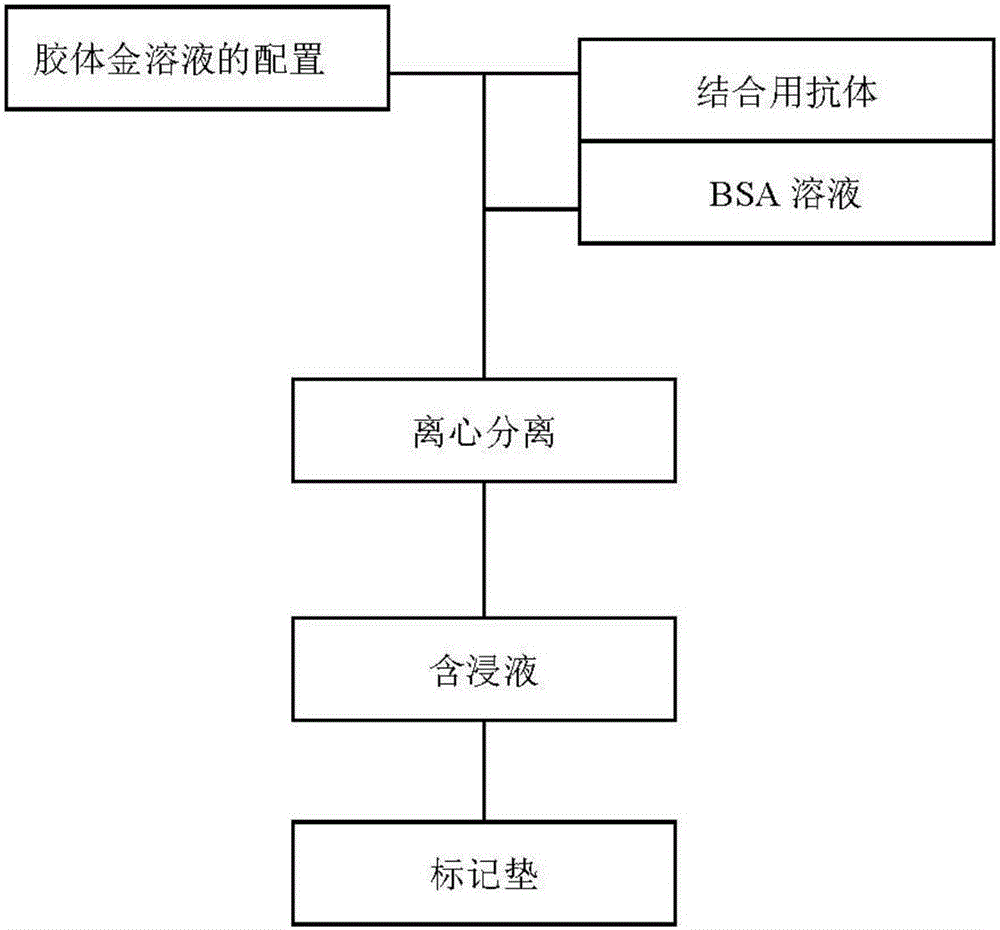
[0039] (1) Preparation of colloidal gold
[0040] The chemical reduction method is used to prepare the required colloidal gold particles by adding trisodium citrate reducing agent into the chloroauric acid aqueous solution. Method First, heat 500ml of 0.01% HAuCL4 solution to boiling, then quickly add 8.0ml of trisodium citrate aqueous solution, heat until red color appears, and then continue to boil for 10-15min. Restore volume to 500ml.
[0041] (2) Colloidal gold-antibody conjugate preparation
[0042] a. Take 500ml of colloidal gold solution, stir it with a magnetic stirrer, and use 0.2M K 2 The CO3 solution adjusted the pH to 8.0.
[0043] b. Dilute the mouse monoclonal antibody against respiratory syncytial virus (Tuno Company, Japan) to 1.5mg / ml with 0.01MPBS, take 5 centrifuge tubes and add 1ml colloidal gold solution respectively, add 30ul, 40ul, 45ul, 50ul of the antibody to be...
Embodiment 2
[0064] The detection application of embodiment 2 kit
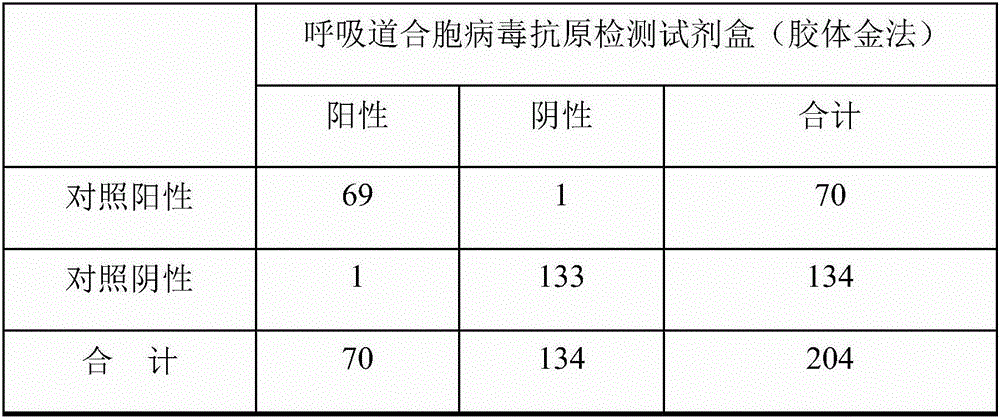
[0065] This kit can qualitatively detect virus samples in nasal swabs, throat swabs, and nasal aspiration samples; through the detection of clinical samples, the coincidence rate of positive and negative is high, and it has high sensitivity and specificity.
[0066] 1180 clinical samples from patients with unknown source of infection were tested in three different hospitals, of which 410 were positive and 770 were negative.
[0067] Sensitivity: 399 positive samples were detected out of 410 positive samples, the sensitivity was 97.3%. Among them, 97 samples of respiratory syncytial virus, 114 samples of adenovirus, 85 samples of influenza A virus (FluA), and 103 samples of influenza B virus (FluB) were detected.
[0068] Specificity: 760 negative samples were detected out of 770 negative samples, the specificity was 98.7%.
Embodiment 3
[0069] Example 3: comparative study of adenovirus detection
[0070] Using the principle of immunochromatography, qualitative detection of adenovirus antigen in throat swab samples. Completely insert the sterilized cotton swab into the throat from the mouth, and rub it several times around the reddened part of the posterior pharyngeal wall and palatine tonsil to collect the mucosal epidermis. When collecting, be careful not to touch saliva.
[0071] Add 0.5ml of sample extraction solution (to the lower reference line) to the attached extraction tube in advance, and place it on the stand. Dip the sterilized cotton swab after collecting the sample into the sample extract in the extraction tube and stir. From the outside of the extraction tube, squeeze the sterilized cotton swab several times with your fingers to fully soak the sterilized cotton swab with the sample extract, then pull out the sterilized cotton swab, and the twisted liquid is used as the sample to be tested. Sque...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com