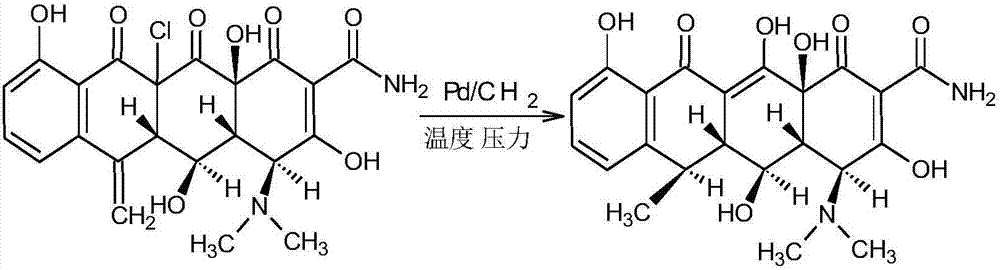
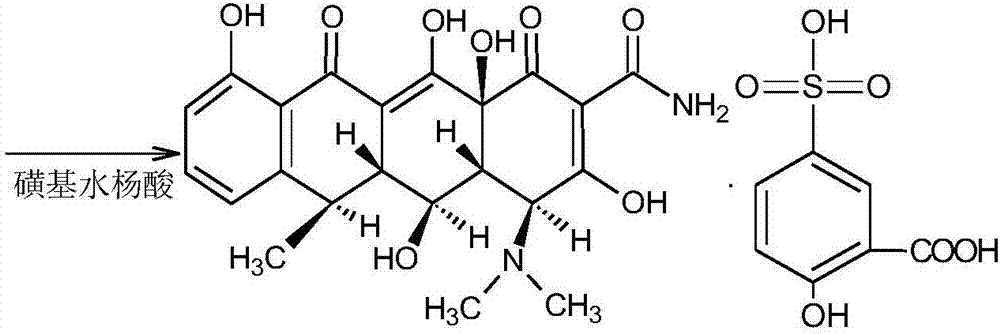
Preparation method of doxycycline hydrochloride intermediate hydride
A technology of doxycycline hydrochloride and preparation process, which is applied in the preparation of organic compounds, the preparation of sulfonates, the preparation of carboxylic acid amides, etc., can solve the problem that the recovery difficulty of sodium tosylate and sodium sulfosalicylate is increased, and the production Cost increase and other problems, to achieve the effect of reducing the cost of three waste treatment, reducing the difficulty of recycling, and improving the recovery rate and product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
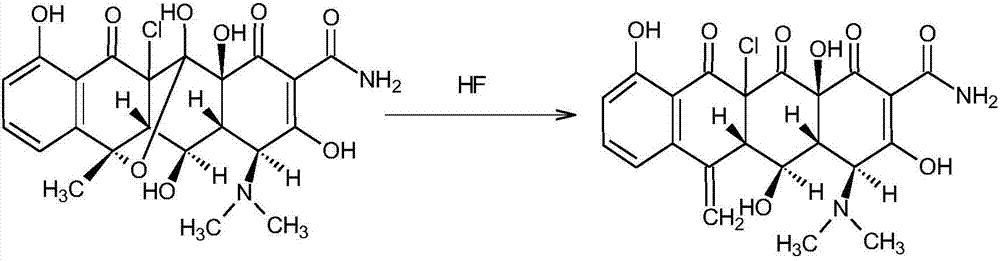
[0027] Oxytetracycline generates chlorinated products after chlorination reaction, and the chlorinated products are air-dried to remove methanol. Inhale 100 kg of dried chlorinated substances into the dehydration reaction pot, prepare 150 kg of HF in the dehydration reaction pot according to the weight ratio of HF:chlorinated compounds at 1.5:1, and pre-cool it to below -20°C with a frozen brine jacket. Stir while inhaling the chlorinated compound, control the reaction temperature after absorbing the chlorinated compound to be 5-10°C, continue to stir and react for 8 hours, stop stirring, and let stand for 6 hours. The dehydrated feed liquid after the reaction is concentrated by a falling film evaporator to remove HF. The concentrated liquid can be received with 200L of methanol in the receiving tank, and the 200L of methanol is pre-cooled to below 0°C with a frozen brine jacket, and then received while stirring, and the temperature of the methanol solution receiving the conce...
Embodiment 2
[0032] Oxytetracycline generates chlorinated products after chlorination reaction, and the chlorinated products are air-dried to remove methanol. Inhale 100 kg of dried chlorinated substances into the dehydration reaction pot, prepare 200 kg of HF in the dehydration reaction pot according to the weight ratio of HF:chlorinated compounds at 2:1, and pre-cool it to below -20°C with a frozen brine jacket. Stir while inhaling the chlorinated compound, control the reaction temperature after absorbing the chlorinated compound to be 0-5°C, continue to stir and react for 10 hours, stop stirring, and let stand for 8 hours. The dehydrated feed liquid after the reaction is concentrated by a falling film evaporator to remove HF. The concentrated liquid is received with 300L methanol in the receiving tank, and the 300L methanol is pre-cooled to below 0°C with a chilled brine jacket, and then received while stirring, and the temperature of the methanol solution receiving the concentrated liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com