Carborane derivative and preparation method and application thereof
A derivative and carborane technology, applied in the field of carborane derivatives and their preparation, can solve the problems of limiting the development of organic single-molecule white light devices, low luminous efficiency, and difficulty in realizing white light, and achieve excellent thermal stability, Efficient white light emission, effect of high thermal decomposition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment designs following compound:
[0034]
[0035] The synthetic route is as follows:
[0036]
[0037] Compound 1-ph: Compound 9-4-ethynylphenanthrene (278.0mg, 1.0mmol), [B 10 h 12 ·(Et 2 S) 2 ] (450.0mg, 1.5mmol, 1.5 times equivalent), 50mL of toluene, stirred evenly in water under oxygen-free conditions, heated to 140°C, and reacted for 72 hours. After the reaction was cooled to room temperature, 20 mL of methanol was added, stirred at room temperature for 1 hour, and then the solvent was removed under reduced pressure. The residue was separated and purified by silica gel column chromatography (petroleum ether / dichloromethane=10:1, v / v), and dried in 258.0 mg of the target product was obtained as a white solid, with a yield of 65%. 1 H NMR (500MHz, CDCl 3 )δ8.79(d, J=8.2Hz, 1H), 8.73(d, J=8.3Hz, 1H), 7.88(d, J=7.7Hz, 1H), 7.81(d, J=8.2Hz, 1H) ,7.73–7.65(m,2H),7.67–7.59(m,4H),7.57–7.48(m,3H),4.05(s,1H,cage-H),3.20–1.70(m,10H). 11 B NMR (160MH...
Embodiment 2
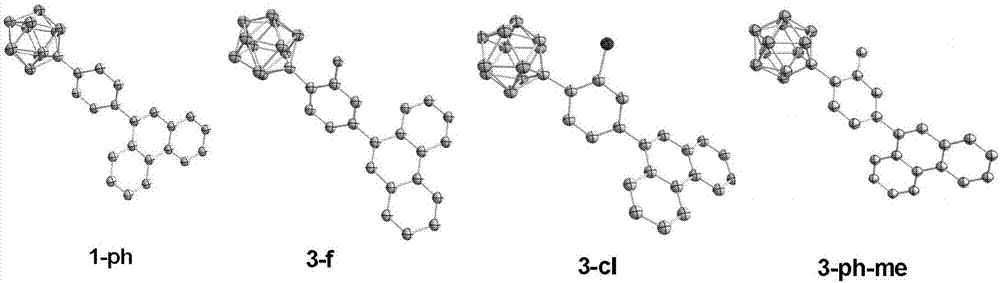
[0043] Use the diffraction intensity data of the compound prepared in Example 1 to be collected on the CCD-Bruker Smart APEX II, use the SHELXTL-97 or SHELXTL-2014 program to analyze the structure, and use the Diamond software to draw a molecular thermodynamic ellipsoid with a probability of 30% (such as figure 1 shown). The results show that compounds 1-ph, 3-cl, 3-ph-me and 3-f have a large steric hindrance in the caged carborane in the crystal structure, which allows the molecules to effectively avoid the π... The π stacking ensures the high luminous efficiency of the compound in the solid state.
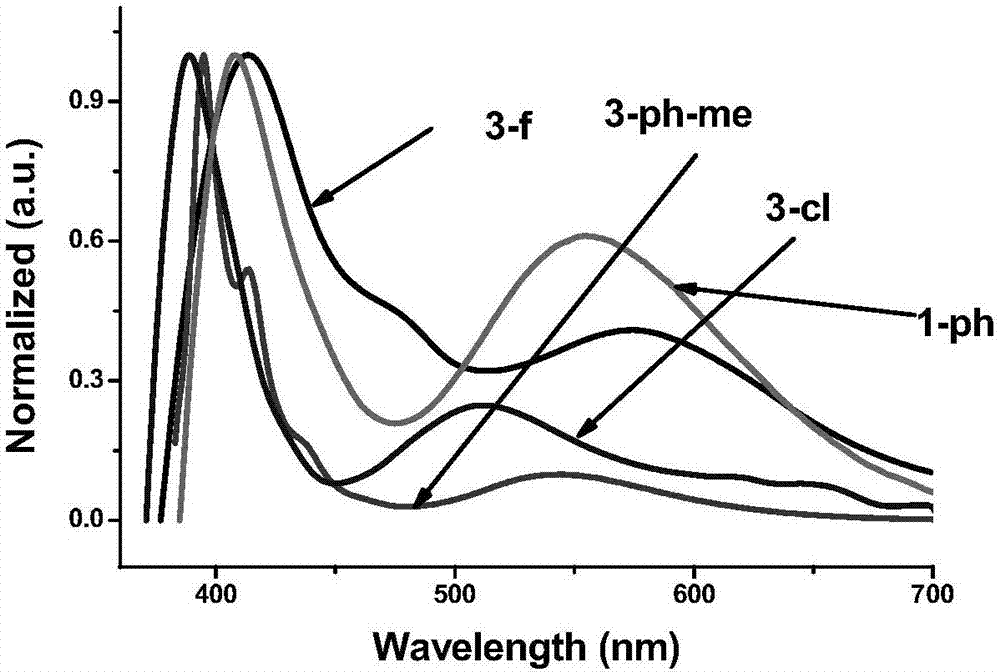
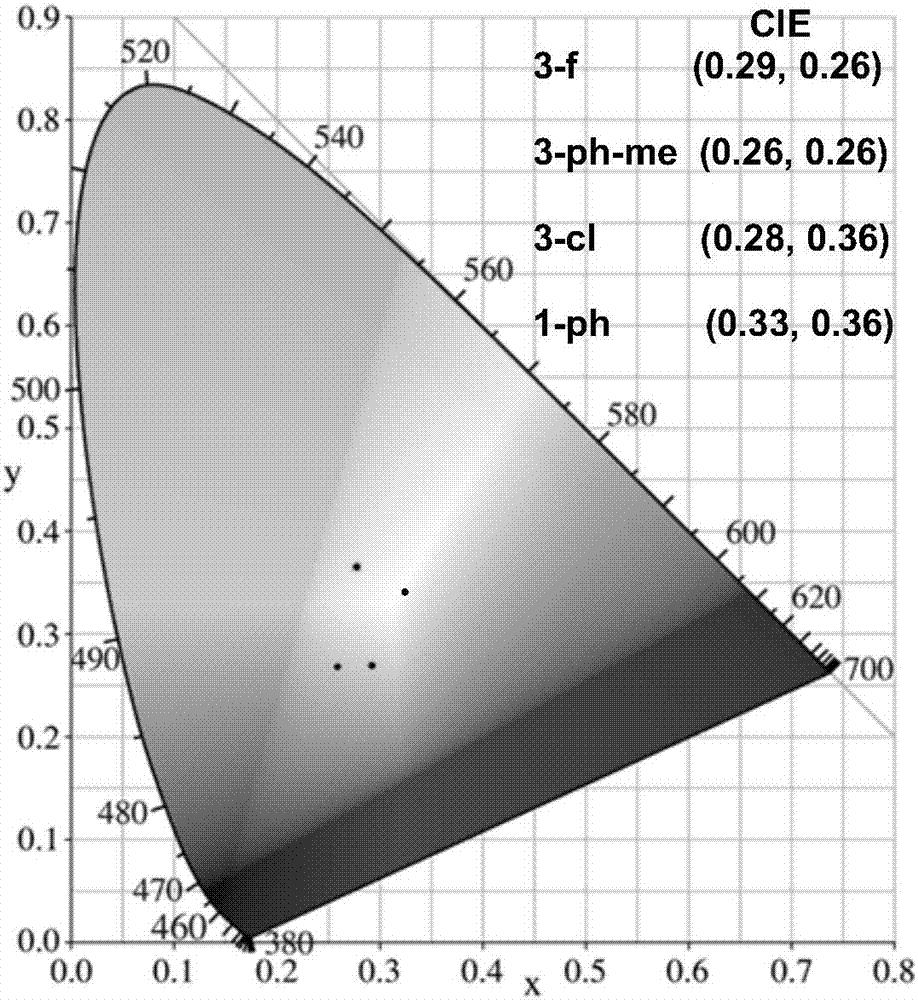
[0044] The emission spectrum of the compound prepared in Hitachi F-4600 Test Example 1 (wherein the absolute fluorescence quantum yield is measured by an integrating sphere, and the quantum yield is: 1-ph, 46%; 3-cl, 17%; 3- ph-me, 50%; 3-f, 67%). The result is as figure 2 As shown, compounds 1-ph, 3-cl, 3-ph-me and 3-f show bimodal emission (380-450, 450-700nm), and are whit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com