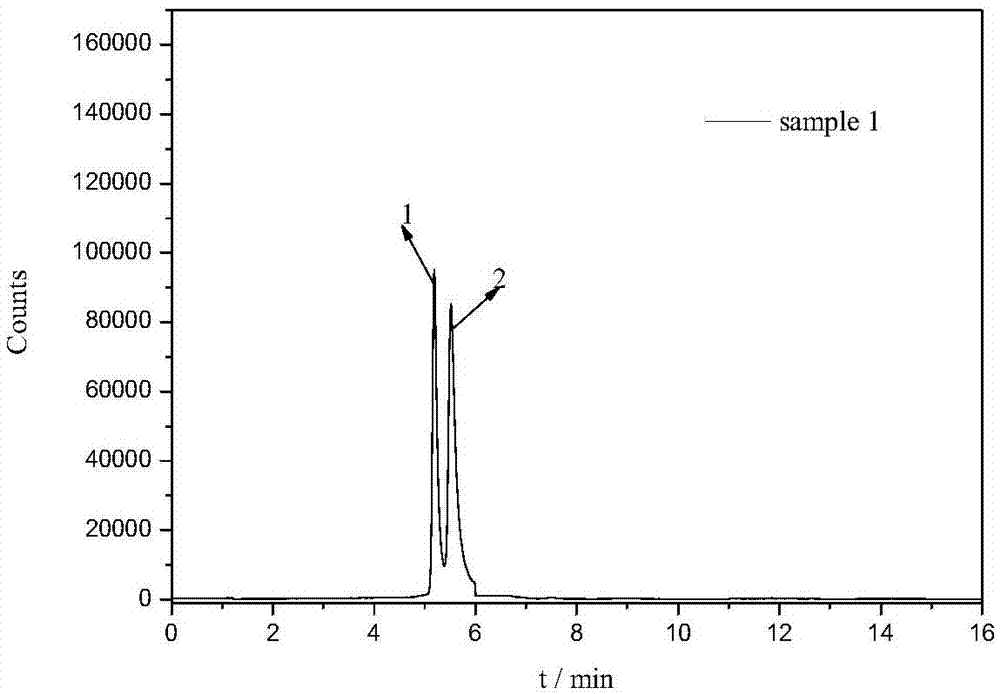
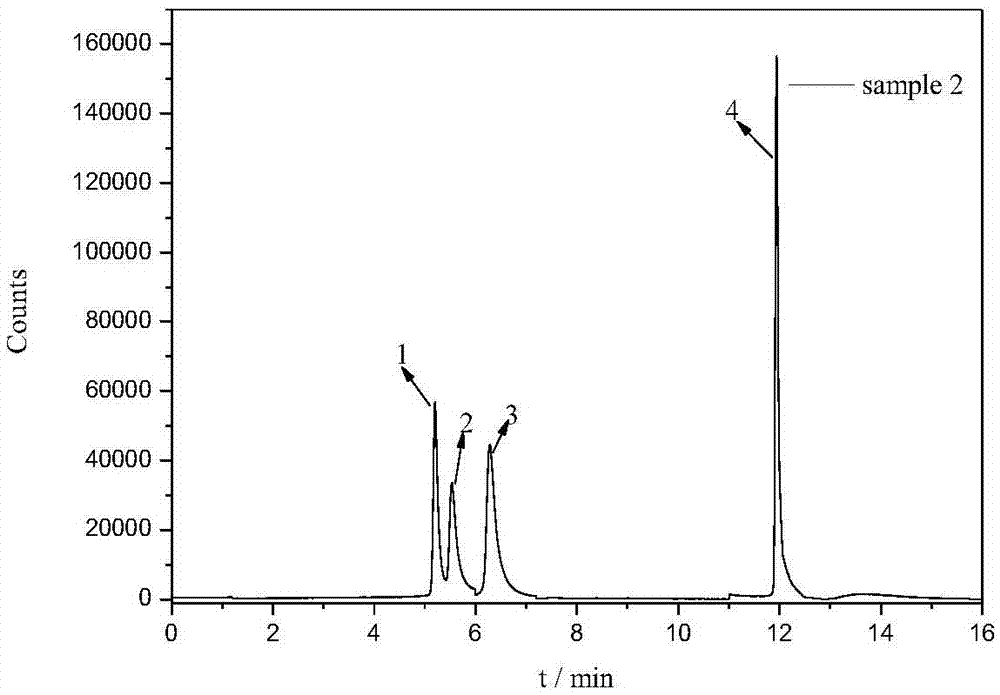
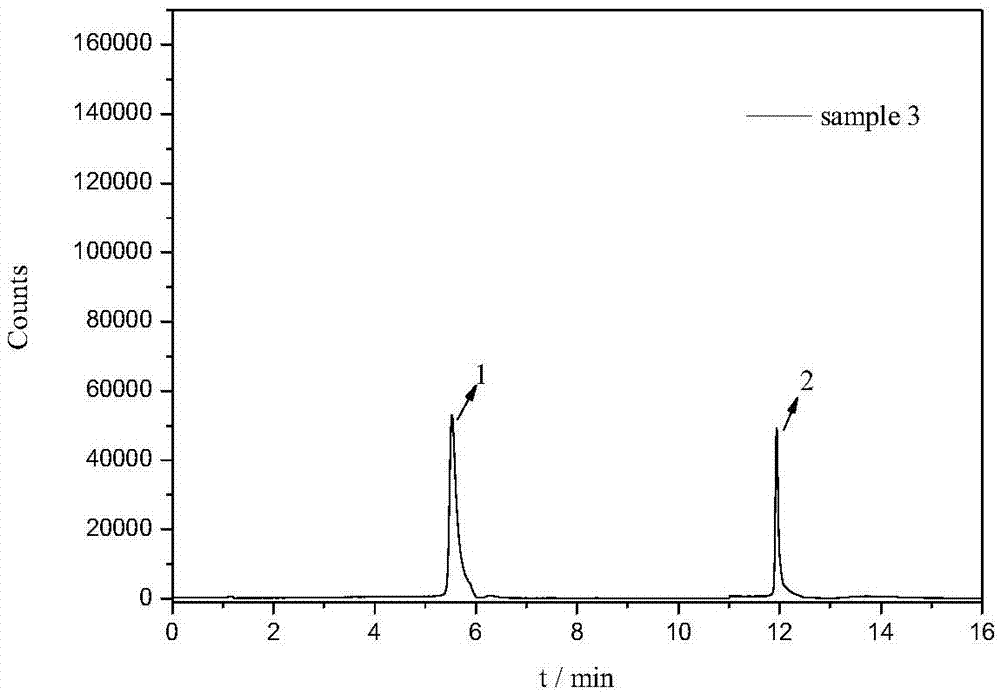
Detection method of tetracycline antibiotics in water sample
A technology of tetracyclines and detection methods, applied in the direction of measuring devices, instruments, scientific instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, the preparation of standard solution
[0037] Six kinds of 1000mg·L -1 Standard stock solution of tetracycline antibiotics: take epitetracycline (ETC), oxytetracycline (OTC), tetracycline (TC), demeclocycline (DMC), chlortetracycline (CTC), anhydrotetracycline (ATC) respectively ) each 10mg (accurate to 0.0001g) in a 10mL brown volumetric flask, dissolved in methanol to the mark, and shake well. All standard stock solutions were stored in a dark, -4°C refrigerator for later use.
[0038] 1000μg·L -1 Mixed standard stock solution of six tetracycline antibiotics: 1000mg·L -1 Standard stock solutions of epitetracycline (ETC), oxytetracycline (OTC), tetracycline (TC), demeclocycline (DMC), chlortetracycline (CTC), and anhydrotetracycline (ATC) were diluted step by step with methanol to 10mg·L -1 , to obtain standard stock solutions of epitetracycline (ETC), oxytetracycline (OTC), tetracycline (TC), demeclocycline (DMC), chlortetracycline (CTC) and anhydro...
Embodiment 2
[0039] Embodiment 2, sample pretreatment
[0040] 100mL water sample was passed through a 0.45μm filter membrane to remove suspended solids, and about 1.5mg of magnetic material was added, heated and stirred in a 40°C constant temperature water bath for 15min for magnetic solid-phase extraction, and the magnetic material after extraction was collected using a magnet, and the extracted magnetic material was discarded. watery. Then use 10 mL ammonia water (pH=11) to sonicate for 10 min for elution, collect the eluate under the action of a magnet, filter through a 0.45 μm filter membrane, and obtain the filtrate for use.
Embodiment 3
[0041] Embodiment 3, linear dependence and sensitivity of the method
[0042] According to the method described in Example 1, an appropriate amount of six tetracycline mixed standard solutions (1000 μg L -1 ), diluted with methanol to obtain mass concentrations of 1, 5, 10, 20, 50 and 100 μg L -1 The series of standard solutions, experiments show that six tetracyclines in 1 ~ 100μg L -1 The linear relationship between the peak area (y) and the mass concentration (x) of the quantitative ion within the mass concentration range is good, and the correlation coefficient r 2 ≥0.995, the limit of detection (LOD, S / N=3) and limit of quantification (LOQ, S / N=10) ranges are 2.44~25.21ng·L, respectively -1 and 8.14~84.03ng·L -1 . (See Table 2)
[0043] Table 2 Linear regression equation, correlation coefficient, detection limit and quantification limit of six tetracyclines
[0044] tetracycline antibiotics linear regression equation Correlation coefficient (r 2 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com