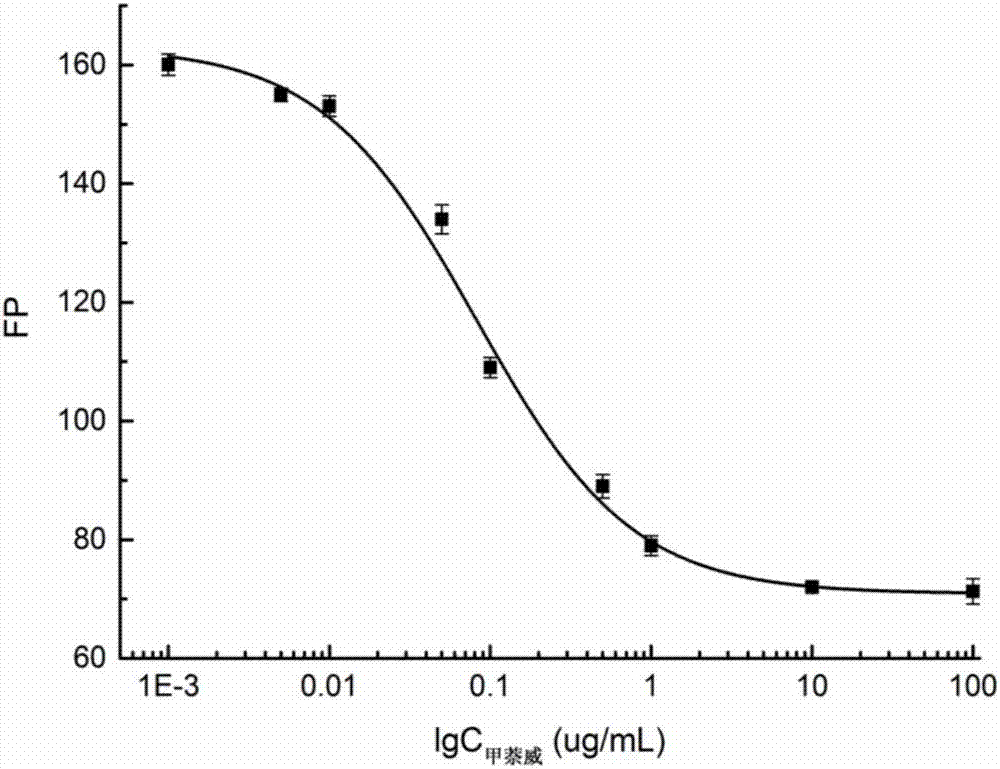
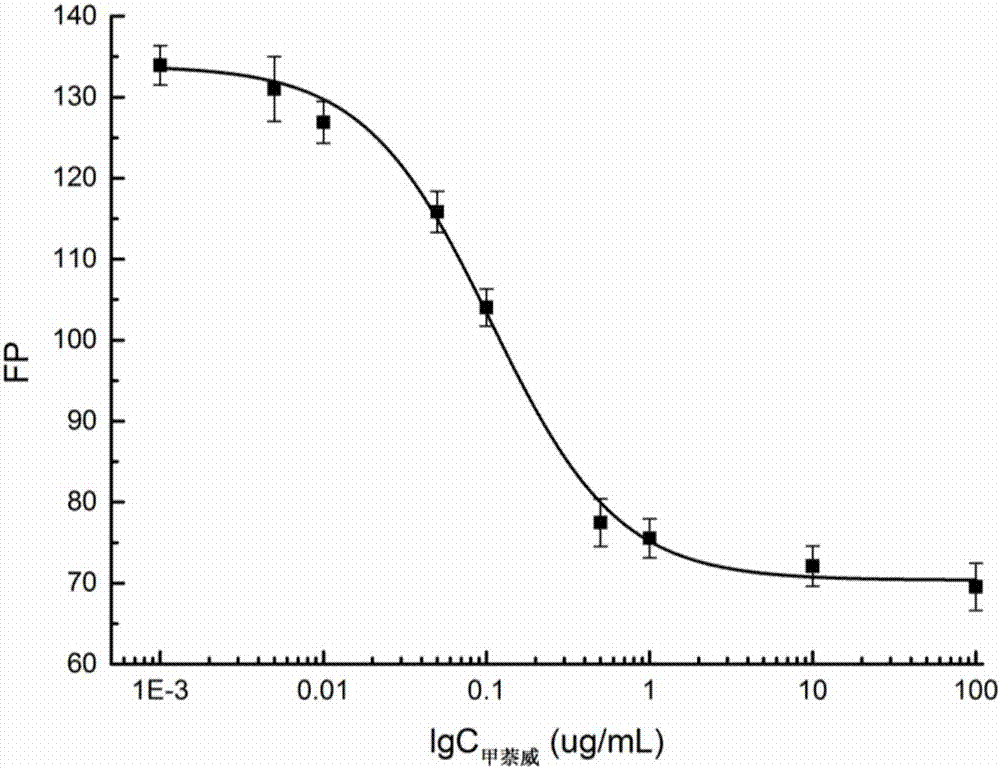
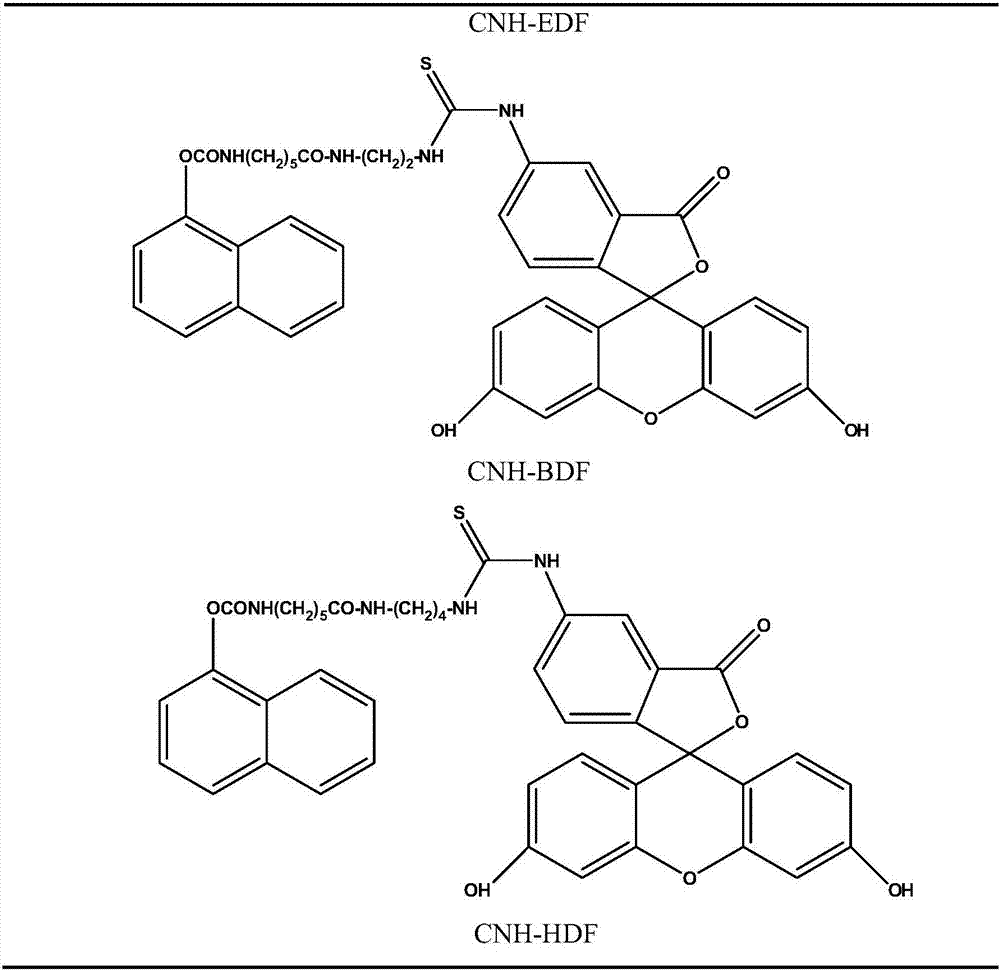
Fluorescence polarization immunoassay method for detecting carbaryl
A fluorescence polarization and immunoassay technology, applied in the field of immunoassay technology and pesticide residue detection, can solve the problems of complex operation and long time consumption, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] 5. Preparation, purification, subtype and identification of anti-carbaryl monoclonal antibody
[0047] The obtained anti-carbaryl monoclonal antibody hybridoma cell line Jnw1D2 was injected into BALB / c mice treated with incomplete Freund's adjuvant in advance, the ascites of the mice was collected, and the antibody was purified by octanoic acid-ammonium sulfate method, specifically The operation is: filter mouse ascites with double-layer filter paper, centrifuge at 12,000 r / min at 4°C for more than 15 minutes, absorb the supernatant, mix the obtained ascites supernatant with 4 times the volume of acetate buffer, slowly add n-octanoic acid while stirring, The volume of n-octanoic acid required per milliliter of ascitic fluid is 30-35 μL, mixed at room temperature for 30-60 min, and allowed to stand at 4 °C for more than 2 h. Centrifuge at 12000r / min at 4°C for more than 30min, discard the precipitate, filter the obtained supernatant with double-layer filter paper, add 1 / ...
example 1
[0050] Example 1, the preparation of fluorescent markers
[0051] Step 1: Preparation of carbaryl hapten synthesis intermediate (1-naphthyloxy-4-nitrobenzene carbonate)
[0052] Install an electric stirrer in a 1L four-necked bottle, configure a thermometer and a constant pressure dropping funnel, add 240mL of dichloromethane, 33mL of triethylamine, and 31.7g of methylnaphthol at room temperature, stir and dissolve, then drop to 0°C with a low-temperature reaction bath ; 40.2g phenyl p-nitrochloroformate was dissolved in 60mL of dichloromethane solution, slowly added dropwise to the above solution, white smoke was produced, and the color change of the solution became darker along with the addition. After 1h dropwise addition, Insulation reaction for 3 hours, TLC monitors the complete reaction of the raw materials (developing agent: dichloromethane: petroleum ether = 1:3); add 360mL of 3% hydrochloric acid, stir for about 30min, separate the liquids, combine the organic phases,...
example 2
[0061] Example 2, Screening of the best fluorescent markers
[0062] Step 1: First, set the working concentration of each fluorescent marker to the concentration (5nM) of the corresponding fluorescent marker when the fluorescence intensity is 10 times the background fluorescence intensity of the borate buffer solution. According to 1 / 125, 1 / 250, 1 / 500, 1 / 1000, 1 / 2000, 1 / 4000, 1 / 8000, 1 / 16000 and 1 / 32000 dilution, draw the antibody binding curve to obtain the maximum value of the signal intensity change δmP (δmP=mP max -mP min ), where the signal change value of CNH-EDF is the largest. The experimental results are shown in Table 2:
[0063] Table 2 The signal intensity of three fluorescent markers combined with antibodies
[0064]
[0065] Step 2: First, the working concentration of each fluorescent marker is set to the concentration (5nM) of the corresponding fluorescent marker when the fluorescence intensity is 10 times the background fluorescence intensity of the BB b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com