Fluorescence polarization immunoassay method for detection of erythromycin
A technology of fluorescence polarization and erythromycin, which is applied in the analysis of materials, fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of complicated operation and long time consumption, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
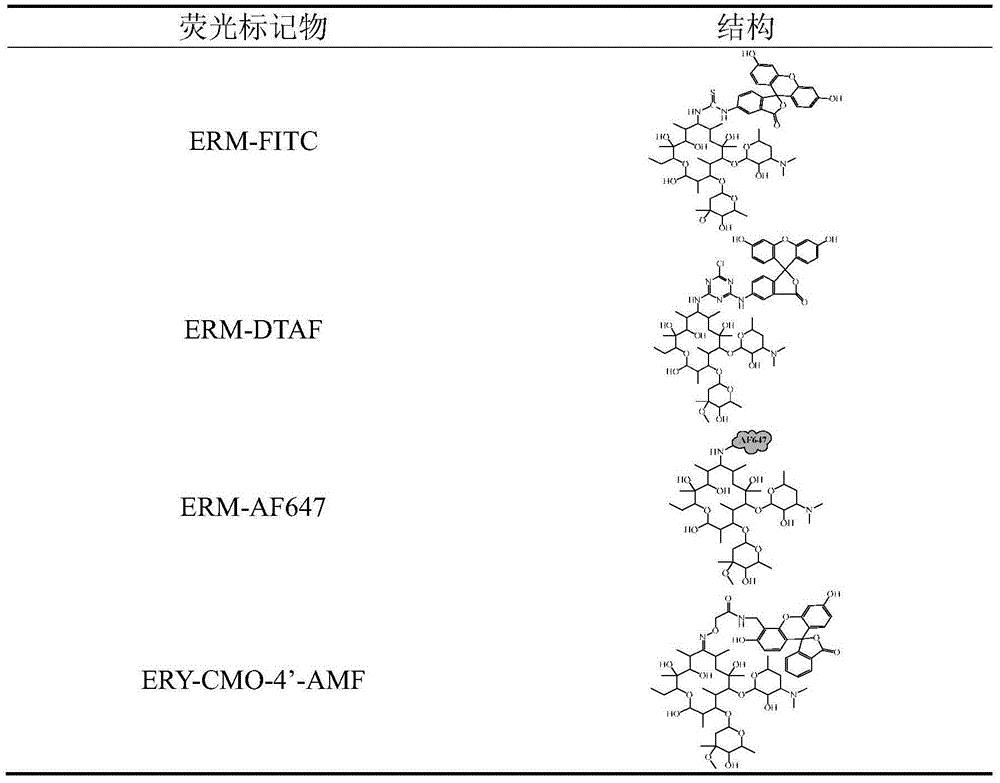
[0039] Example 1, preparation of fluorescent marker based on hapten erythromycinamine
[0040] Taking ERM-FITC as an example, the synthesis method is as follows:
[0041] Weigh 0.2mg of erythromycin standard substance and 1mg of FITC powder, add to 200μL of DMF and shake until dissolved; add 50μL of triethylamine, and react in the dark at room temperature for 7 days; take 50μL of the reaction solution and separate it with thin layer chromatography (TLC) to obtain pure FITC For comparison, the developer is dichloromethane / methanol (v:v, 1:1); scrape off the silica gel plate to scrape R f = 0.5 yellow band, eluting with methanol, ready for detection. After identification by mass spectrometry, the peak m / z of the ERM-FITC product was 1123.4.
[0042] The reaction steps of other fluorescent markers of erythromycylamine such as DTAF, tetramethylrhodamine sulfonyl chloride, and AF647 are similar to those of FITC, and the fluorescent markers are stored at 4°C.
example 2
[0043] Example 2, Preparation of fluorescent marker based on modified erythromycin hapten
[0044] Step 1: Modification of erythromycin hapten
[0045] will contain 44mg carboxymethylhydroxylamine NaHCO 3Adjust the pH to between 5-6 and add 2 mL of distilled water dropwise to 1 mL of absolute ethanol containing 100 mg of erythromycin raw material; put it in a constant temperature water bath at 50°C and stir for 5 hours; after the reaction, cool naturally to room temperature and add water to quench , adjust the pH of the solution to be acidic; extract twice with dichloromethane, combine the dichloromethane layers, add anhydrous Na 2 SO 4 Shake and stand overnight; take the dichloromethane layer, 40°C rotary evaporation to dryness under reduced pressure, dissolve with 5mL DMF, and store at -20°C.
[0046] Step 2: Synthesis of modified erythromycin fluorescent marker (ERY-CMO-4'-AMF)
[0047] Weigh 200 μL ERY-CMO DMF solution and mix with 200 μL DMF solution containing 0.4 mg...
example 3
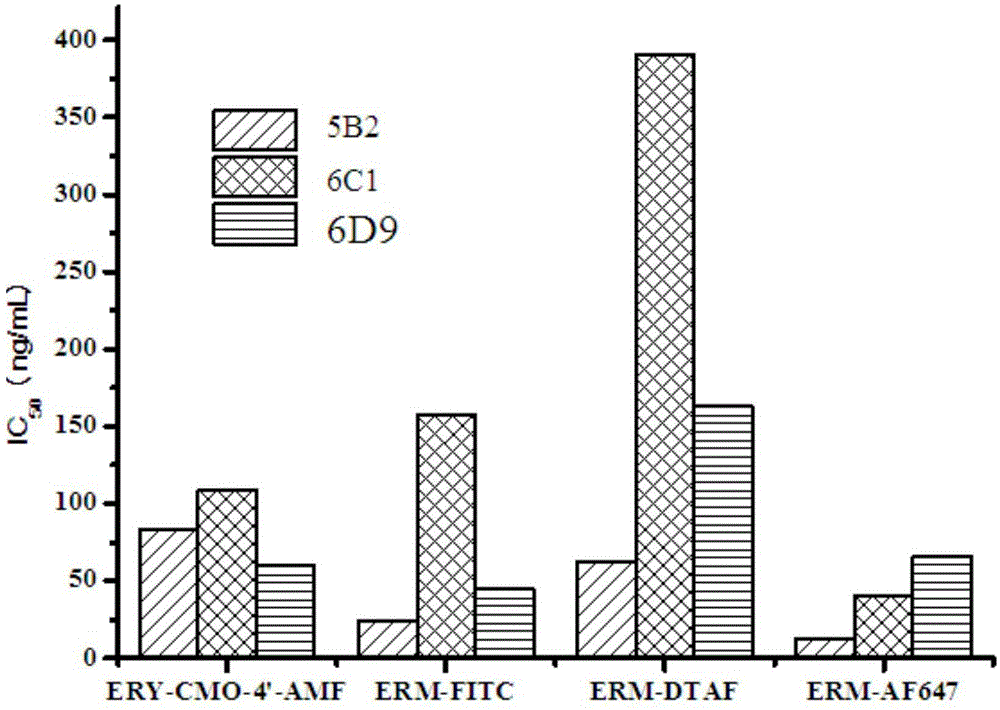
[0048] Example 3. Screening of the best fluorescent marker and antibody combination
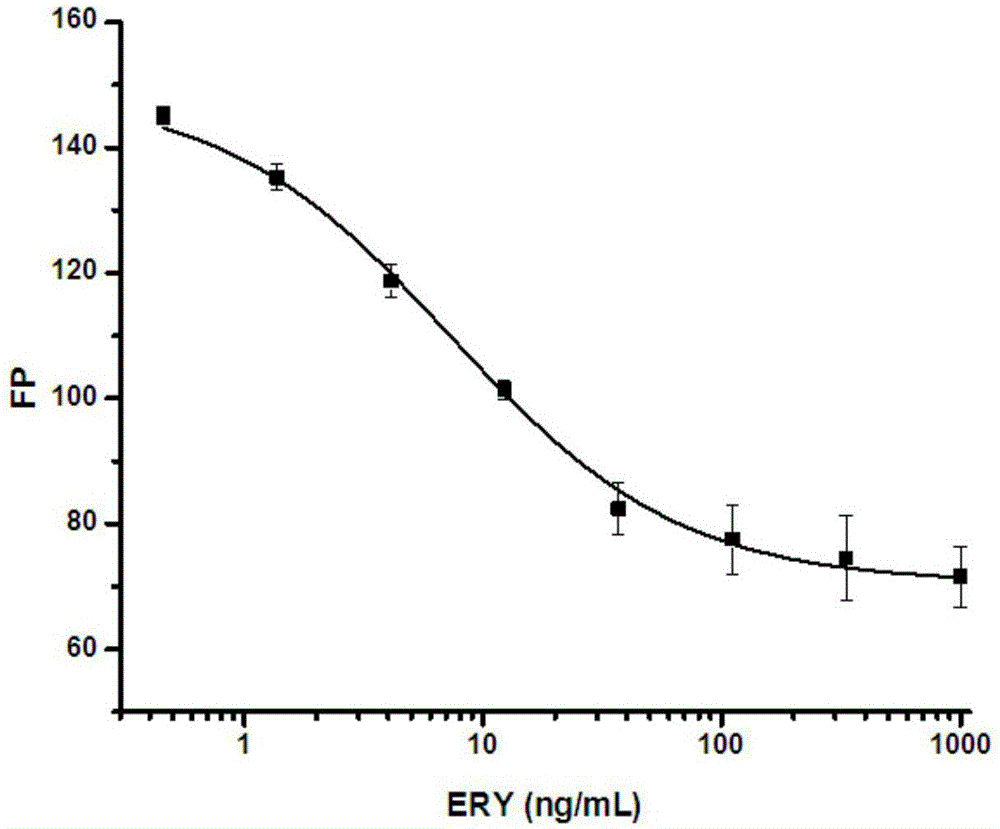
[0049] Step 1: Combining various synthetic fluorescent markers with three erythromycin monoclonal antibodies (5B2, 6C1 and 6D9), first set the working concentration of each fluorescent marker so that the fluorescence intensity is the background of the buffer solution The concentration of the fluorescent marker corresponding to 10 times the fluorescence intensity (15nM), each antibody with borate buffer solution according to 1 / 100, 1 / 200, 1 / 400, 1 / 800, 1 / 1600, 1 / 3200, Dilute 1 / 6400, 1 / 12800, 1 / 25600 and 1 / 51200, draw the antibody binding curve, and obtain the maximum change value of signal intensity δmP (δmP=mP max -mP min ), (ERM-FITC, ERM-DTAF and ERY-CMO-4'-AMF excitation wavelength 485nm, emission wavelength 530nm, cutoff value 515nm; ERM-AF647 excitation wavelength 644nm, emission wavelength 685nm, cutoff value 665nm) Among them, ERM- FITC had the greatest signal change value. The expe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com