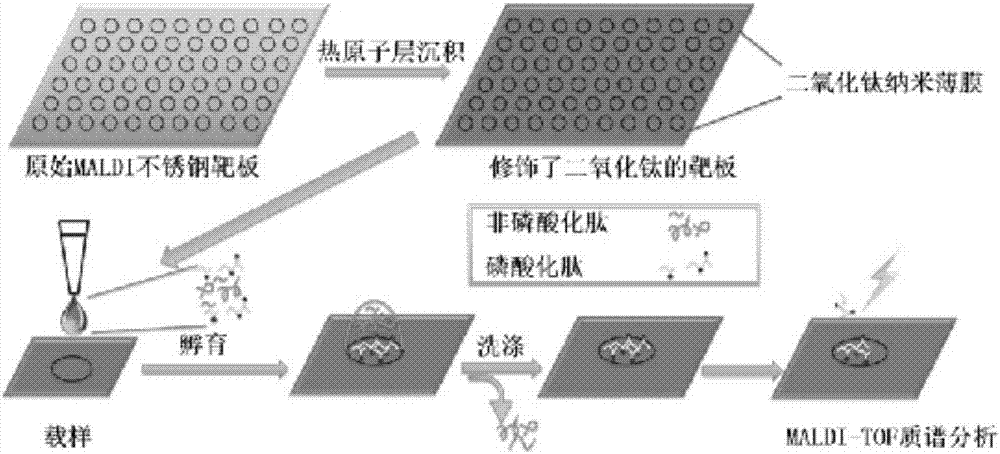
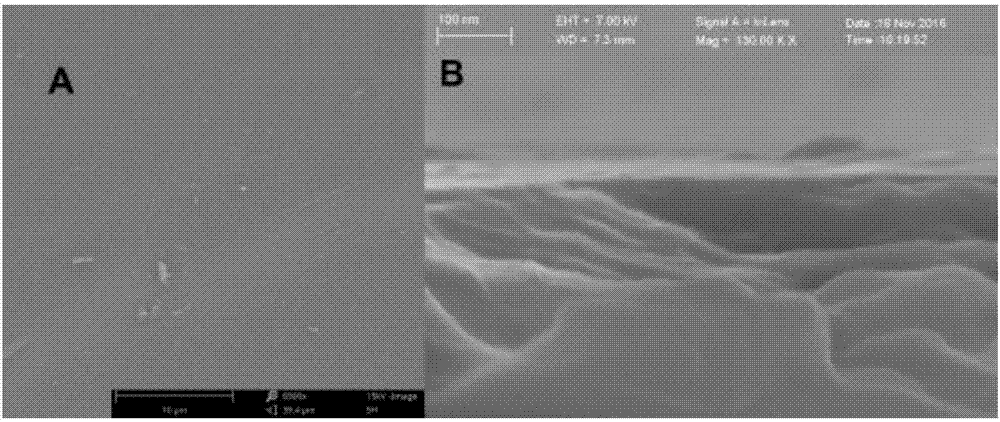
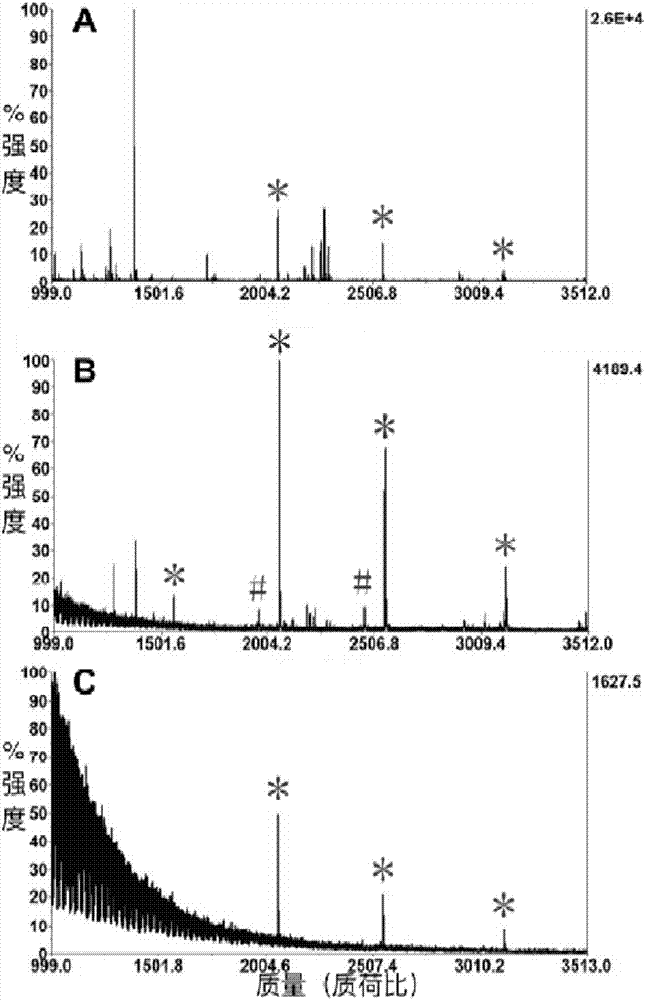
Method for modifying titanium dioxide nanofilm on target plate and application thereof
A nano-film, titanium dioxide technology, applied in the field of MALDI target plate, can solve the problems of inability to guarantee consistency, limited firmness of enrichment material and target plate, ion source pollution, etc., to achieve simple and time-saving enrichment process and long storage time. , good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of a target plate for modifying titanium dioxide nano film.
[0030] (1) Wipe the MALDI target plate to be modified with a dust-free paper dipped in an appropriate amount of detergent, and then ultrasonically clean it with 1% formic acid solution, ethanol solution and deionized water for 10 minutes each, and then clean it again with deionized water. Rinse and dry;
[0031] (2) Put the target plate obtained in step (1) into an atomic layer deposition system, using tetrakis(dimethylamino)titanium (TDMAT) and deionized water as titanium source and oxidation source, respectively. In the reaction chamber, the MALDI target plate was alternately exposed to TDMAT and deionized water, and high-purity argon was used as the carrier gas and purge gas. For all samples, one cycle during deposition consisted of a 0.2 s TDMAT pulse, a 10 s rinse, a 0.2 s deionized water pulse, and a 10 s rinse. The desired film thickness is achieved by varying the number of cyc...
Embodiment 2
[0034] Example 2: Application of titanium dioxide modified target plate on the target to purify phosphorylated peptides in β-casein enzymatic hydrolysis solution.
[0035] (1) Preparation of standard protein enzymatic hydrolysis solution: Accurately weigh standard protein β-casein and BSA and dissolve them in 25 mM ammonium bicarbonate buffer solution respectively, so that the protein concentration is 1 mg / mL, boil for 10 minutes, after cooling, follow the enzymatic Add an appropriate amount of trypsin (trypsin) at a ratio of 1:40 to the protein, and digest at 37°C for 16 hours.
[0036] (2) Purification of phosphorylated peptides on the target and MALDI analysis: Rinse and dip the modified target plate with enrichment buffer (50% acetonitrile aqueous solution containing 0.1% trifluoroacetic acid) for 1 minute for activation and then dry it. Then dilute the β-casein hydrolysis solution with enrichment buffer to an appropriate concentration, and pipette 0.8 μL onto the modified...
Embodiment 3
[0038] Example 3: Investigation of the selectivity of purified phosphorylated peptides on the target.
[0039] (1) Preparation of mixed protein enzymolysis solution: prepare a mixture of β-casein and BSA enzymolysis solution with a molar ratio of 1:200, dilute to an appropriate concentration with enrichment buffer (make the concentration of β-casein 400 fmol / uL ), used to investigate the selectivity of purified phosphorylated peptides from the target plate.
[0040] (2) Investigation of the selectivity of purified phosphorylated peptides on the target: Rinse and dip the modified target plate with enrichment buffer (50% acetonitrile aqueous solution containing 0.1% trifluoroacetic acid) for 1 minute for activation and then dry it. Use a pipette gun to pipette 1 μL of the mixture onto the modified target plate, and then place the target plate in a wet box with a lid for static incubation for about 10-30 minutes. Afterwards, the target plate was quickly and fully washed several ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com