A method of improving stability of a perovskite oxide cathode
A perovskite oxide, perovskite technology, applied in electrochemical generators, fuel cells, electrical components, etc., can solve the problems of reduced battery performance and reduced oxygen reduction sites, so as to improve battery performance and improve cathodes The effect of degradation and stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
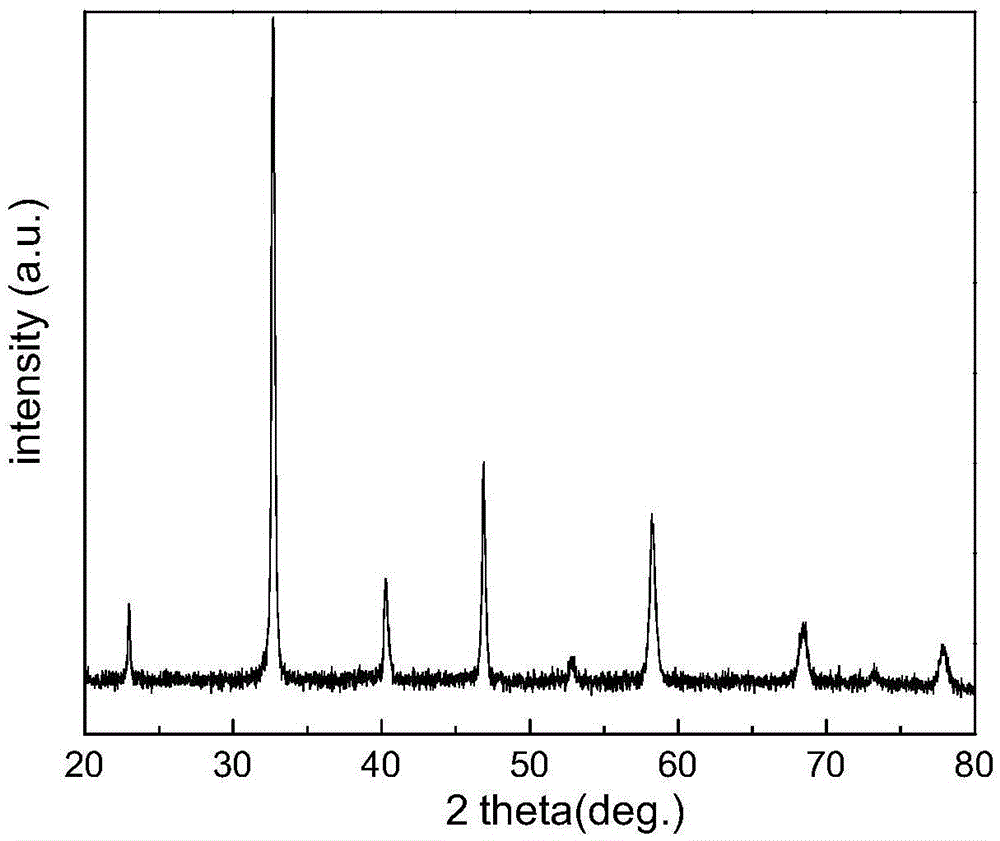
[0019] La was synthesized by ammonium citrate method 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3-ζ Cathode powder, where La 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3-ζ Take 0.05mol. Weigh 12.9872gLa(NO 3 ) 3 ·6H 2 O (analytical pure), 4.2325gSr (NO 3 ) 2 (analytical pure), 2.9105gCo(NO 3 ) 2 ·6H 2 O (analytical pure), 16.16gFe (NO 3 ) 3 9H 2 O (analytical pure) was added into a beaker with deionized water and stirred to dissolve it completely. Then add 36.483g ammonium citrate (analytical pure) according to the ratio of ammonium citrate: metal ion molar ratio of 1.5:1, adjust the pH=9 of the solution with ammonia water (analytical pure) to make the solution clear and transparent, then heat and stir to evaporate the solvent Pour the solution into an evaporating dish until the solution becomes viscous and sol-like, heat it with an electric furnace to make the system self-propagating and burn, collect the primary powder and roast it in a muffle furnace at 1000°C, and use XRD to character...
Embodiment 2
[0021] Co-synthesis of La by ammonium citrate method 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3-ζ / Gd 0.1 Ce 0.9 o 1.95 =50:50wt% composite cathode impregnated nanoparticles, where Gd 0.1 Ce 0.9 o 1.95 Take 0.025mol. Weigh 0.4531gGd 2 o 3 Powder (analytical pure), in a 250ml beaker, add HNO 3 (analytical pure) after stirring to dissolve it completely, weigh 6.5486g Ce(NO 3 ) 3 ·6H 2 O (analytical pure), 5.0409gLa (NO 3 ) 3 ·6H 2 O (analytical pure), 1.6429gSr (NO 3 ) 2 (analytical pure), 1.1297gCo(NO 3 ) 2 ·6H 2 O (analytical pure), 6.2724gFe (NO 3 ) 3 9H 2 O (analytical pure) was added into a beaker with deionized water and stirred to dissolve it completely. Then according to ammonium citrate: the ratio of metal ion mol ratio is 1.5:1 to add 23.2814g ammonium citrate (analytically pure), adjust the pH=1 of solution with nitric acid (analytical pure) to make the solution become clear and transparent, and the solution is settled at 100ml volumetric flask.
Embodiment 3
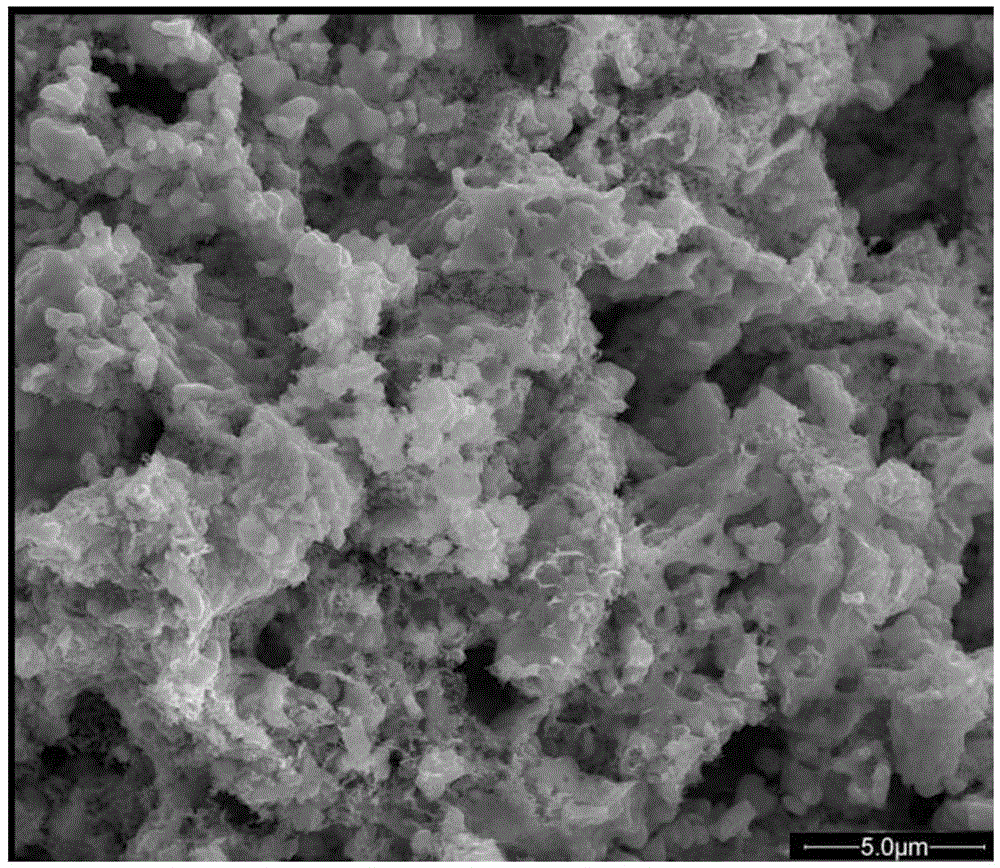
[0023] La prepared as in Example 1 0.6 Sr 0.4 co 0.2 Fe 0.8 o 3-ζ Powder, after grinding, add 50wt% cathode paste (the composition of the added glue is a terpineol (90wt%) solution that dissolves ethyl cellulose (10wt%).) Configure the cathode slurry, and coat 0.0080g to Gd 0.1 Ce 0.9 o 1.95 After sintering on the interlayer at 900°C for 2 hours, use a microliter syringe to take 3uL of the solution prepared in Example 2 and dip it into La 0.6 Sr 0.4 co 0.2Fe 0.8 o 3-ζ In the cathode, bake at 800°C for 2 hours to make the impregnating solution phase, and then test it on the self-assembled battery evaluation device at 700°C, 1A / cm 2 Constant current discharge at current density. figure 2 SEM image of the cross-section of the cathode after firing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com