Lithium battery electrolyte and lithium battery
An electrolyte and lithium battery technology, applied in the field of electrochemistry, can solve the problems of easy battery swelling, affecting the charging and discharging efficiency and life of lithium ion batteries, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 11
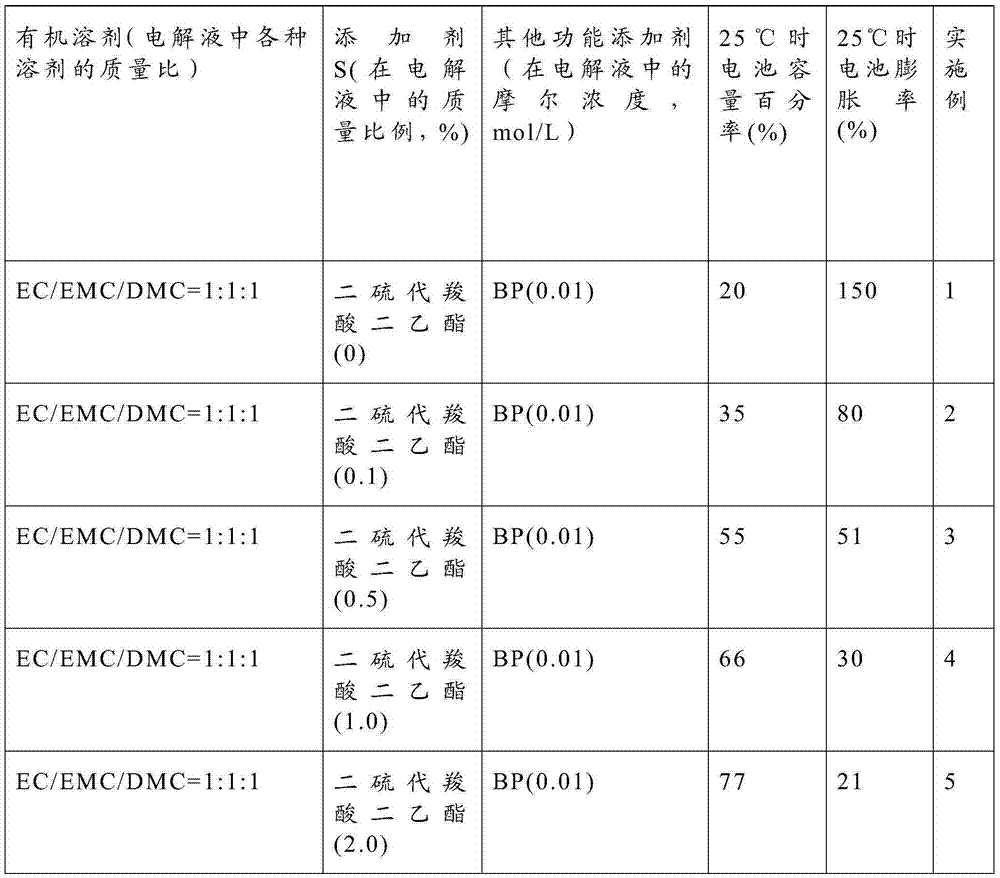
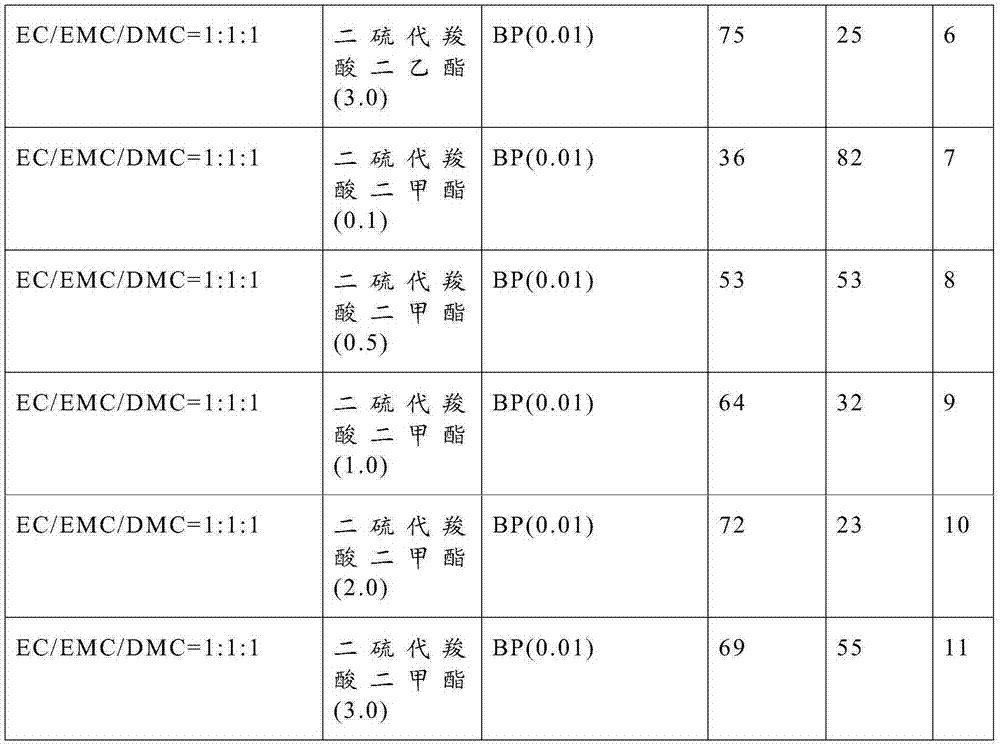
[0024] Electrolyte: the lithium salt is lithium hexafluorophosphate, and the molar concentration of the lithium salt is 1 mol / liter. See Table 1 for other components and dosages in the electrolyte.
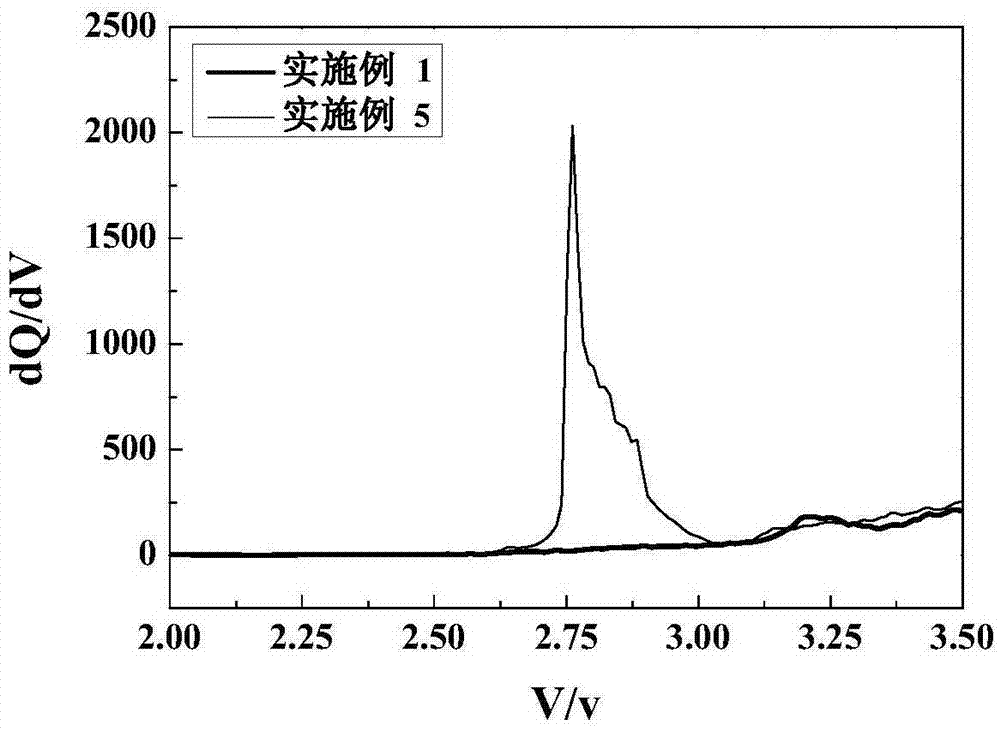
[0025] Negative electrode: artificial graphite, positive electrode: lithium cobalt oxide material, electrolyte, positive electrode, negative electrode are assembled into a lithium battery according to the conventional process, and the coulombic efficiency comparison of the initial charge of the lithium battery prepared in Example 1 and Example 5 can be found in figure 1 , Test the percentage of battery capacity and the battery expansion rate of the lithium battery prepared in each embodiment after 300 cycles of charging and discharging, the results are shown in Table 1.
[0026] Table 1
[0027]
[0028]
[0029] From figure 1 It can be seen that the embodiment 5 containing diethyl dithiocarboxylate forms a passivation film on the negative electrode of the lithium-ion batte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com