Construction method of hyperuricemia model
A technology of hyperuricemia and construction method, applied in the field of experimental model construction, can solve the problems of skin and mucous membrane liver damage, hyperuric acid instability, long-term maintenance of hyperuricemia model, avoiding animal damage, and the method is safe and reliable. , the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: The specific steps of the specific construction of hyperuricemia model in this embodiment are as follows
[0022] (1) Animal sample selection and model construction: 8-week-old male clean-grade Wistar rats with a body mass of (200±20) g were selected, purchased from Shandong Lukang Pharmaceutical Co., Ltd., and fed to SPF-grade animals of Qingdao University School of Medicine. Breeding room. Before the start of the experiment, normal rat pellet feed was used to feed adaptively for 1 week. During the experiment, the rats’ body surface characteristics were observed, whether there were abnormal changes in behavioral activities, and the abnormal rats were isolated. 40 Wistar rats with no abnormalities in appearance characteristics and behavioral activities; the environment for adaptive feeding is: temperature: 20-24°C, relative humidity: 50%-70%, light rhythm: 12D: 12L, working illumination: 150- 300lx, air flow, wind speed 0.1~0.2m / s, noise≤60dB; The feed of ...
Embodiment 2
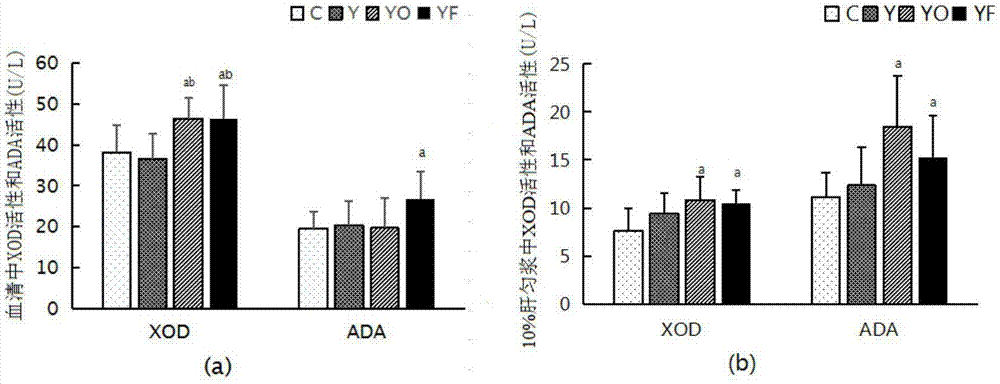
[0026]In this embodiment, 40 male Wistar rats were divided into groups according to body weight by the random number table method, which were respectively blank control, yeast control, yeast oxonate potassium and yeast fructose 4 groups, 10 in each group, and the blank control group was given ordinary rats. Rats were fed with pellet feed, and the other three groups were given yeast feed with a mass fraction of 0.2; the rats in the yeast fructose group were given drinking water with a mass concentration of 10% fructose, and the other three groups were given tap water for drinking; kg·d) Potassium oxonate was gavaged, and the other three groups were gavaged with distilled water, and the volume of gavage was 10ml / (kg·d); all rats were free to drink water and eat; At 8 o'clock in the morning of 8W, the rats were fasted for 12 hours, then cut their tails and took 1.0-1.5ml of blood, left it at room temperature for coagulation, centrifuged at 3000r / min for 5min, took 300μl of serum, ...
Embodiment 3
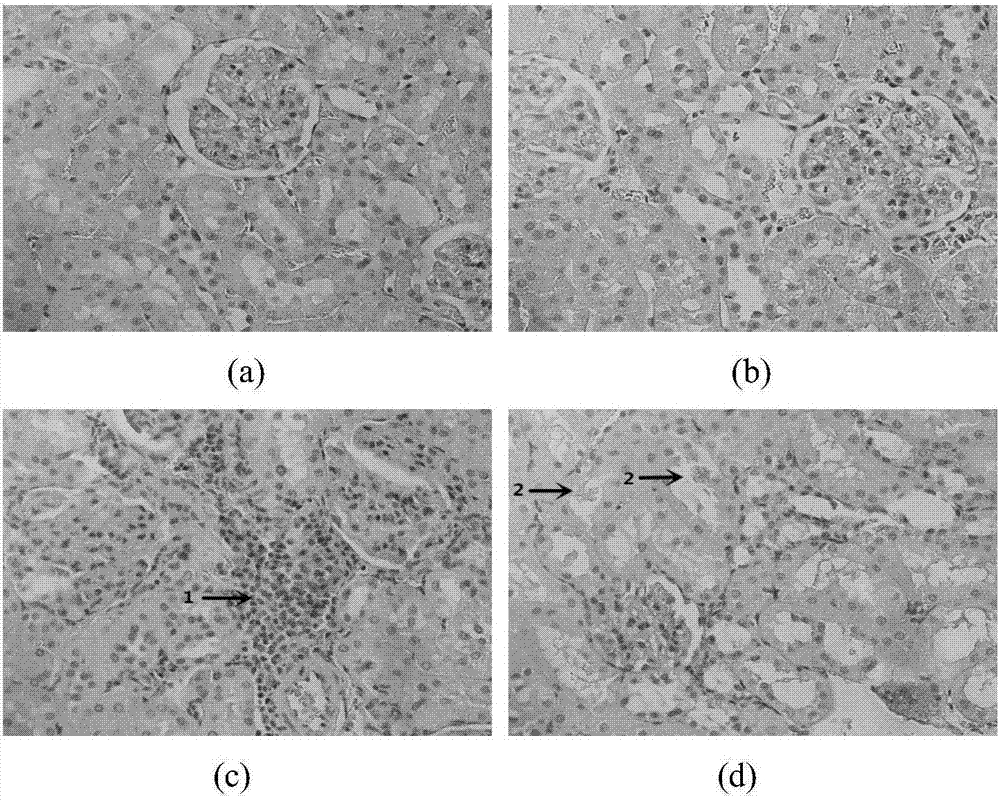
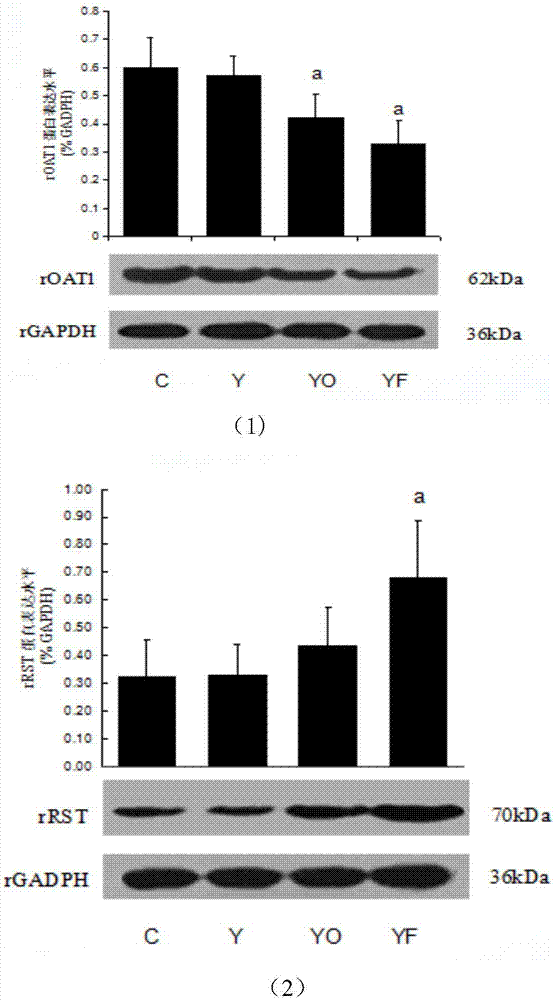
[0041] This example adopts the animal intervention of Example 1 to carry out the experiment: at the end of the 8th week of the animal experiment, after the rats were fasted for 12 hours, the rats were sacrificed, the right kidneys of the rats in each group were removed, fixed with 4% paraformaldehyde, and conventional paraffin wax After embedding, 3 μl thick sections were made, stained with HE, and observed under a light microscope (×400); the results are shown in the attached figure 2 As shown, the kidney structure of the rats in the blank control group and the yeast control group was normal, the glomeruli in the renal cortex were evenly distributed, the size was normal, and there was no swelling and degeneration of the renal tubules; Infiltration of mononuclear lymphocytes, no obvious fibrosis; occasional crystal deposits in the interstitium of renal tubules in yeast fructose group, no obvious fibrosis; comprehensive conclusion: occasional crystal deposits in renal tubules o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com