Application of NADPH (nicotinamide adenine dinucleotide phosphate) in preparation of medicine for treating cardiac hypertrophy and cardiac failure
A technology for myocardial hypertrophy and heart failure, applied in drug combinations, pharmaceutical formulas, cardiovascular system diseases, etc., can solve problems such as increased arrhythmia, many adverse reactions, and general curative effect in the decompensated period, achieving cardiotonic effect and significant The effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
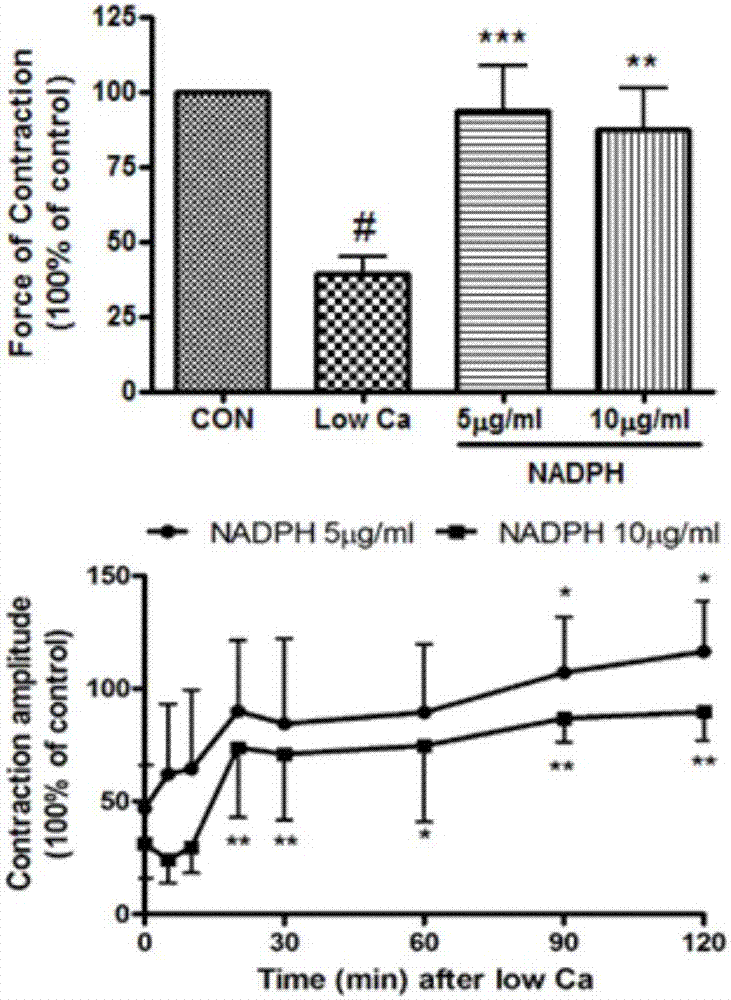
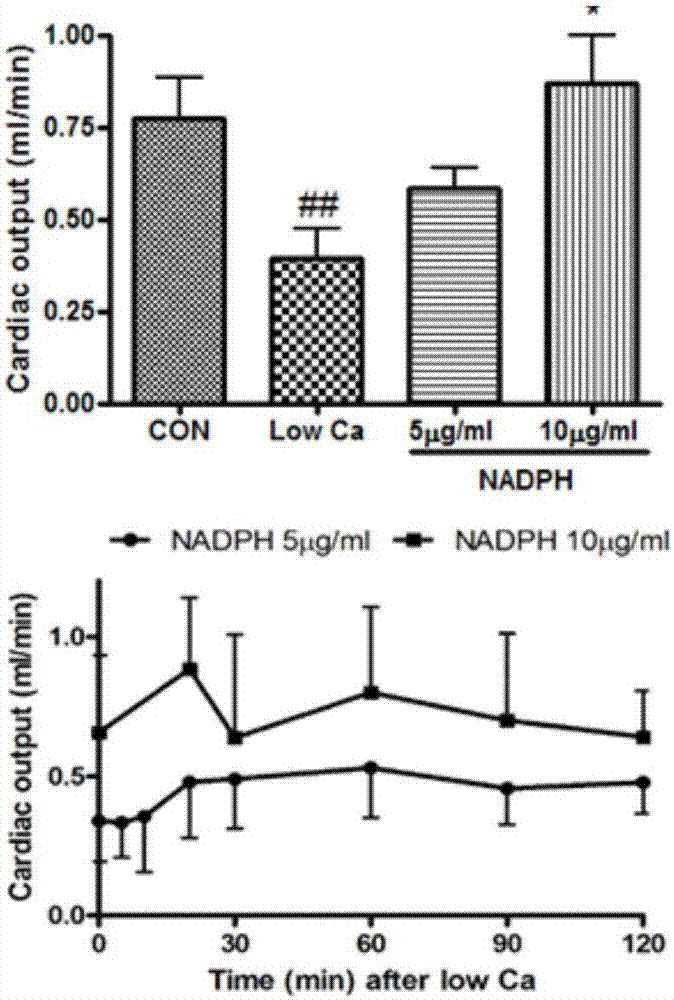
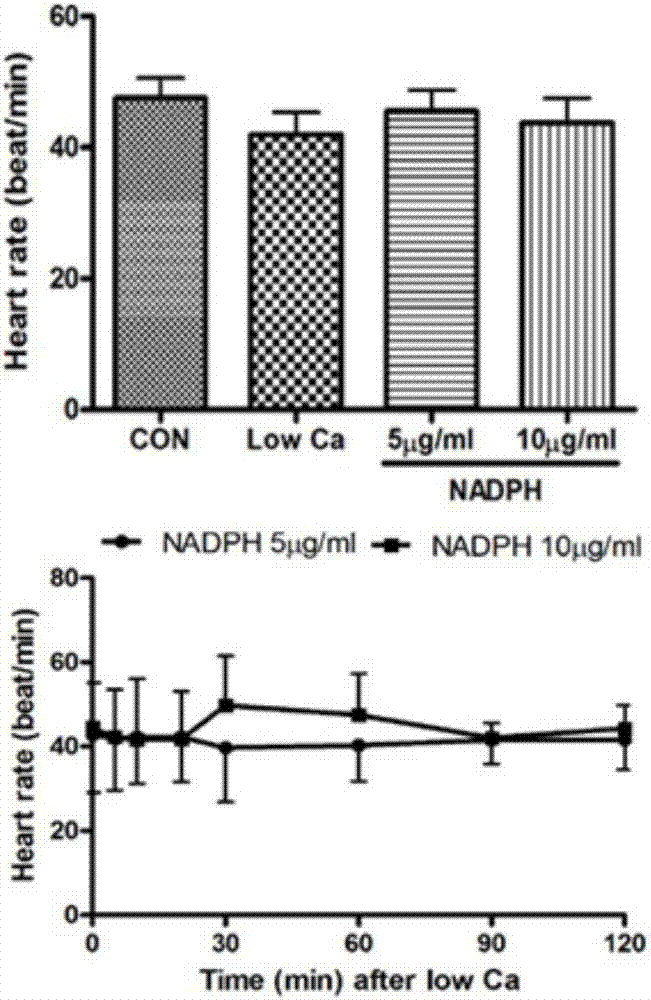
[0028] Experimental example 1 The Experiment of Cardiotonic Effect of NADPH on Frog Heart in Vitro
[0029] (1) Experimental materials
[0030] Toad (60-80g) was provided by the Experimental Animal Center of Soochow University School of Medicine, experimental animal use license number: SYXK (Su) 2002-0037;
[0031] Animal breeding environment: room temperature 22°C, humidity 50-60%, well ventilated, artificial day and night (12h / 12h), free intake of food and water;
[0032] The source of exogenous NADPH drugs can be obtained through artificial synthesis, semi-synthesis, and biological extraction;
[0033] (2) Experimental method
[0034] The toads were demyelinated and fixed on the frog plate, the left and right aortas and inferior vena cava were separated, the inferior vena cava was inserted into the venous cannula, the left aorta was inserted into the arterial cannula, the right aorta was ligated, and Ren's solution (1000mL: NaCl 6.5g , KCl 0.14g, CaCl 2 0.12g, NaHCO ...
experiment example 2
[0040] Experimental example 2 Mouse model of cardiac hypertrophy
[0041] (1) Experimental materials
[0042] SPF grade ICR mice (male, weighing 18-22g) were provided by the Experimental Animal Center of Soochow University School of Medicine, experimental animal production license number: XCYK (Su) 2002-2009;
[0043] Animal breeding environment: room temperature 22°C, humidity 50-60%, well ventilated, artificial day and night (12h / 12h), free intake of food and water;
[0044] The source of exogenous NADPH drugs can be obtained through artificial synthesis, semi-synthesis, and biological extraction;
[0045] ATPase kit (A070-6), purchased from Nanjing Jiancheng Bioengineering Institute;
[0046] Isoproterenol (ISO) and captopril (Cap) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd.;
[0047] Chloral hydrate was purchased from Sinopharm Chemical Reagent Co., Ltd.;
[0048] Electrocardiograph (Kenz, ECG-103).
[0049] (2) Experimental method
[0050...
experiment example 3
[0079] Experimental example 3 Effect of NADPH on Blood Pressure of Normal Rats
[0080] (1) Experimental materials
[0081] SD rats (male, weighing 250-300 g) were provided by the Experimental Animal Center of Soochow University School of Medicine;
[0082] Animal breeding environment: room temperature 22°C, humidity 50-60%, well ventilated, artificial day and night (12h / 12h), free intake of food and water;
[0083] Non-invasive blood pressure detection system (Kent Scientific, CODA20496).
[0084] (2) Experimental method
[0085] Non-invasive measurement of tail artery blood pressure in rats: use volume pressure recording sensor to non-invasively measure the blood pressure of conscious rats. The rats are fed normally for 2 days, and the blood pressure of the rats is measured with a non-invasive blood pressure detection system. The experimental environment is controlled in a quiet and constant temperature, and the adaptive training starts after 3 days formal experiment. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com