Nitrogen mustard compounds containing hydroxamic acid groups as well as preparation method and application of nitrogen mustard compounds
A compound and composition technology, applied in the field of medicine, can solve the problems of inability to completely kill tumor cells and easy to produce drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Synthesis of a part of compounds in the present invention
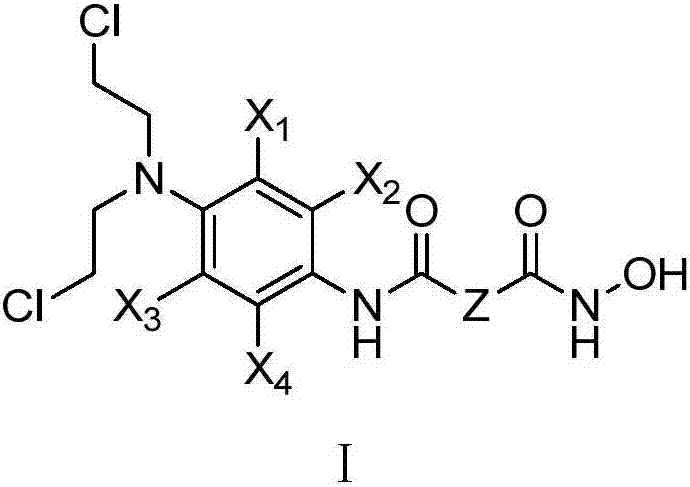
[0048] Preparation of N-{4-[bis(2-chloroethyl)amino]phenyl}-N'-hydroxysuberamide (H101).
[0049] Step (1): Weigh p-fluoronitrobenzene (2.80g, 20mmol) and diethanolamine (10.50g, 100mmol) in a 50ml single-necked round-bottom flask and stir at 118 degrees Celsius, and confirm that the reaction is complete by thin-layer chromatography Afterwards, the reaction solution was cooled to normal temperature, added into ice water under stirring, a large amount of solids were precipitated, and 3.94 g of yellow solids (yield 87%) were obtained by filtration.
[0050] Step (2): Weigh the yellow solid (2.76g, 12.2mmol), dichloromethane (27.5ml) and pyridine (10ml) obtained in the step (1) in a 100ml three-necked round-bottomed flask, and bring the whole reaction system to the Cool to 0°C in an ice bath, add thionyl chloride (3.27g, 27.5mmol) dropwise with a constant pressure dropping funnel, and after confirmi...
Embodiment 2
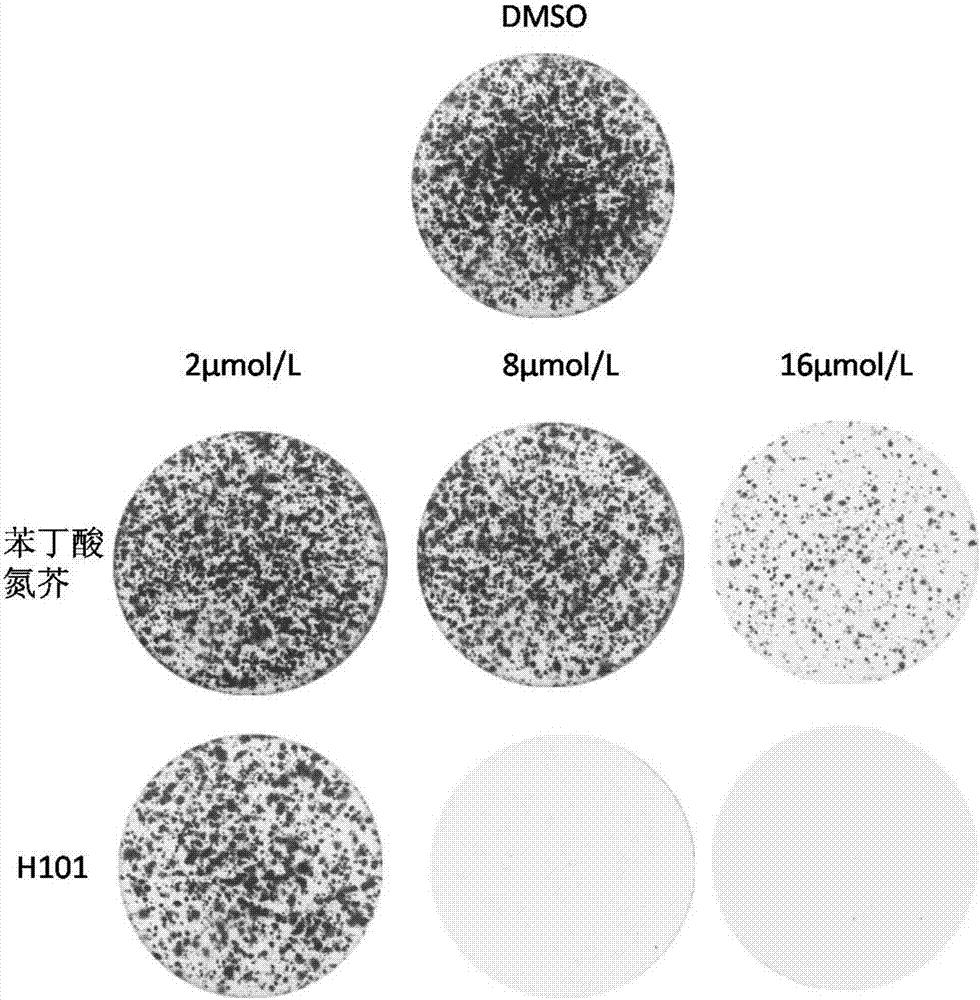
[0060] Example 2: Compound anti-tumor cell proliferation experiment
[0061] (1) Experimental cell lines, five kinds of cancer cells were selected: human skin melanoma A375, human cervical cancer cell HeLa, human liver cancer cell HepG2, human lung cancer cell A549, and human colon cancer cell HCT116. Before the experiment, five kinds of cancer cells were preserved in liquid nitrogen. First, the cancer cells were taken out, and the temperature was rapidly raised to 37 degrees Celsius in a water bath at 37 degrees Celsius. Afterwards, transfer to 24mL cell culture flasks, add 6ml of culture medium to each flask based on 37 degrees Celsius, containing 5% CO 2cultured in an incubator, (wherein human skin melanoma A375, liver cancer cells HepG2, and human cervical cancer cells HeLa were subcultured after routine digestion with 0.25% trypsin in DMEM medium containing 10% fetal bovine serum. Human lung cancer cells A549 and human colon cancer cells HCT116 were subcultured in RPMI-1...
Embodiment 3
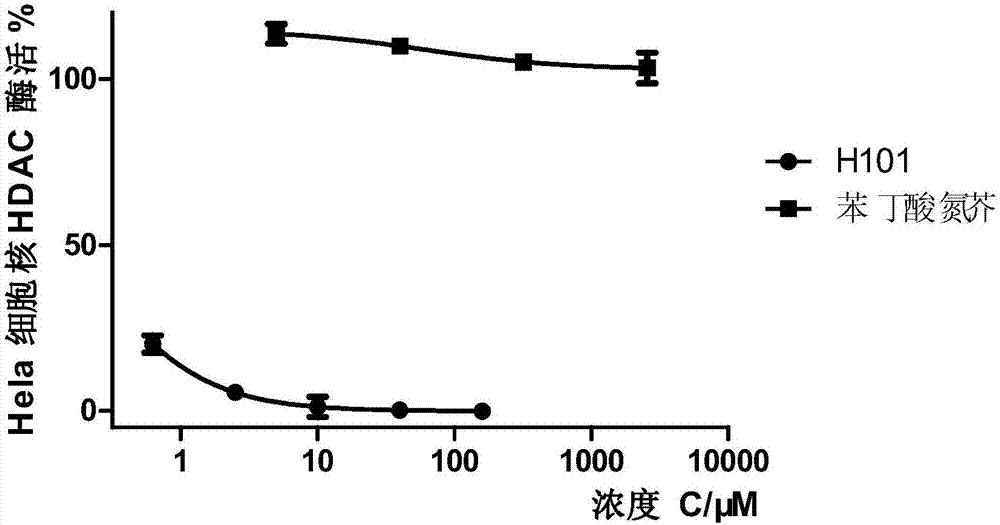
[0066] Embodiment 3: Compound is to the inhibition experiment of HDAC enzyme
[0067] use switzerland The HDAC green fluorescence test kit (product serial number: BML-AK53R) produced by Life Sciences was used for the HDAC enzyme activity inhibition experiment of HeLa cell nucleus extract, and the HDAC1 enzyme activity inhibition experiment was carried out using the kit with the product serial number BML-AK511. The kit with serial number BML-AK512 was used for HDAC2 enzyme activity inhibition experiment, and the kit with product serial number BML-AK516 was used for HDAC6 enzyme activity inhibition experiment. The operation was carried out in strict accordance with the kit instructions, 15 μL of HDAC enzyme was thoroughly mixed with 10 μL of the compound to be tested, and the substrate and the above mixture were placed at 37 degrees Celsius for 5 minutes to obtain the same starting temperature for the substrate and enzyme, and then each well was quickly Add 25 μL of substrate,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com