Method for preparing geranyl acetate under catalysis of acidic functionalized ionic liquid
A technology of geranyl acetate and ionic liquids, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of non-recyclability, environmental pollution, etc., and achieve less side reactions, environmental friendliness, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
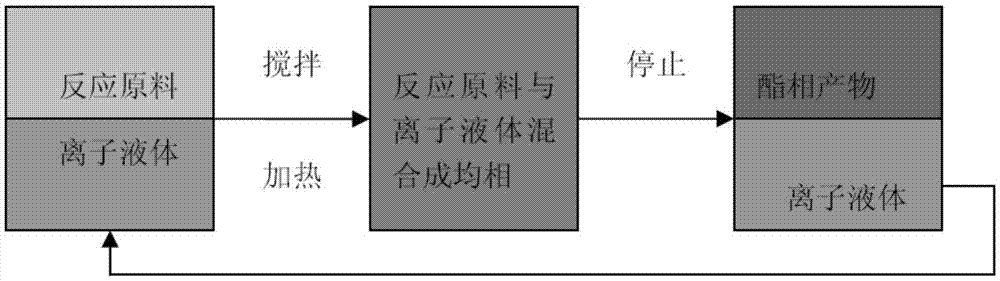
[0029] Take 14.48g of acetic acid and 30.84g of geraniol, mix them evenly and add them into a three-necked flask, add 5g of acidic functionalized ionic liquid catalyst I, heat and reflux and stir, use a water separator to separate water, react at 110°C for 2h, cool it with diethyl ether Extraction, the raffinate is the recovered catalyst, ether is recovered under reduced pressure, and geranyl acetate is obtained by distillation. The yield is shown in Table 1.
[0030] The reaction condition and reaction result of table 1 embodiment 1~5
[0031] Example temperature reflex yield purity 1 90℃ 84% 98.2% 2 100℃ 95% 98.4% 3 110℃ 97% 99.1% 4 120℃ 92% 98.0% 5 130℃ 91% 97.5%
[0032] Wherein, the preparation method of acidic functionalized ionic liquid catalyst I is as follows:
[0033] Mix 1,3-propane sultone and methylethylbutylamine at a molar ratio of 1:1, react at 40°C for 10 hours to obtain a white solid, wash and dry the wh...
Embodiment 6~9
[0035] Catalyst recycling: the catalyst in Example 3 was not treated in any way, and 14.48g of acetic acid and 30.84g of geraniol were added, reacted at 110°C for 2h, extracted with ether after cooling, recovered ether, and distilled under reduced pressure to obtain acetic acid aroma. Leaf ester, the yield is shown in Table 2.
[0036] The catalyst application process is as follows:
[0037] The reaction conditions and reaction result of embodiment 6~9
[0038] Example repeat times yield 3 0 97.0% 6 1 96.8% 7 2 95.9% 8 3 95.5% 9 4 95.2%
Embodiment 10~11
[0040] Different acidic ionic liquid catalysts were used to conduct experiments under the same other conditions. The experimental method was consistent with that of Example 3. The yield and content data are shown in the table below.
[0041] Catalyst type and reaction result of embodiment 10~11
[0042] Example catalyst yield content 10 Ionic Liquid II 95.4% 97.5% 11 Ionic liquid III 92.4% 96%
[0043] The preparation method of ionic liquid II is as follows:
[0044] Mix 1,3-propane sultone and triethylamine at a molar ratio of 1:1, react at 40°C for 10 hours to obtain a white solid, wash and dry the white solid with ethyl acetate. Take 1 equivalent of the above-mentioned white solid and slowly add it to a reaction kettle containing 1 molar equivalent of p-toluenesulfonic acid under an ice-water bath, stir for 2 hours, then raise the temperature to 80°C for 8 hours, wash with ethyl acetate, and distill under reduced pressure to remove Ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com