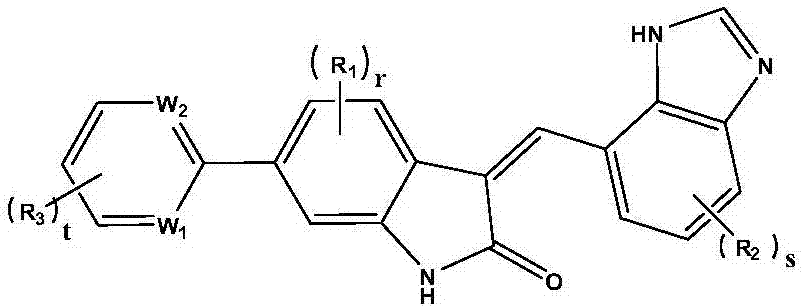
Medicine for preventing and treating coronary heart disease and application thereof
A drug and pharmaceutical technology, applied in the field of indolinone derivatives or their pharmaceutically acceptable salts, can solve the problems of not being able to have a good effect on cardiovascular and cerebrovascular diseases, and achieve the effect of increasing HDL levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
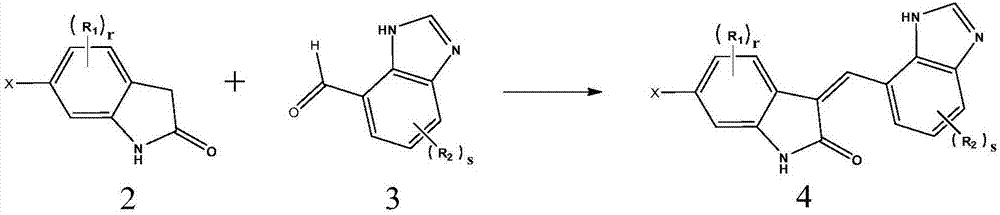
[0062] The present invention also provides a preparation method of the compound shown in formula 1, the preparation method comprising the following steps:
[0063] Step 1. react the compound shown in formula 2 with the compound shown in formula 3 to prepare the compound shown in formula 4, as shown in following reaction formula 1:
[0064] [Reaction 1]
[0065]
[0066] In Equation 1, R 1 , R 2 , r, s are as defined in formula 1, X represents halogen, preferably chlorine or bromine;
[0067] The reaction is carried out in the presence of a base, which includes inorganic bases such as sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, sodium bicarbonate or potassium bicarbonate; and organic bases such as triethylamine, pyridine or piperidine.
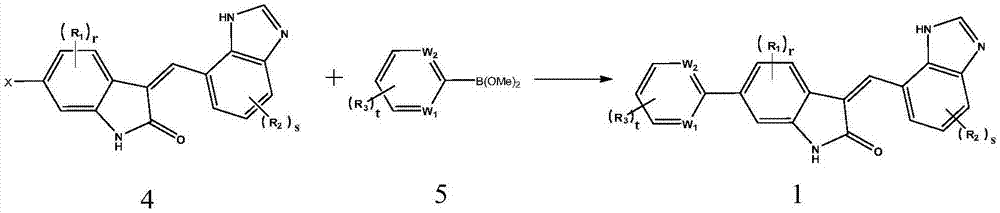
[0068] Step 2. react the compound shown in formula 4 with the compound shown in formula 5 to prepare the compound shown in formula 1, as shown in following reaction formula 2:
[0069] [Reaction 2]
[...
Embodiment 1
[0081] Example 1: (Z)-3-((1H-benzo[d]imidazol-7-yl)methylene)-5-methyl-6-(pyridin-2-yl)indoline-2- Ketone (Compound I)
[0082]
[0083] Step 1: Put 1H-benzo[d]imidazole-7-carbaldehyde (2.19g, 15.0mmol) in a 500ml eggplant-shaped bottle, add 150ml of absolute ethanol to dissolve, then add 6-chloro-5-methylind Indolin-2-one and 2ml triethylamine were reacted at room temperature for 3 hours, and a large amount of precipitation appeared. Suction filtration, washing with a small amount of absolute ethanol, drying to obtain yellow solid (Z)-3-((1H-benzo[d]imidazol-7-yl)methylene)-6-chloro-5-methylindole Lin-2-one 4.15g, yield 89.2%, content 98.1%. ESI-MS: 310.07[M+H] + .
[0084] Step 2: Add (Z)-3-((1H-benzo[d]imidazol-7-yl)methylene)-6-chloro-5-methylindoline-2 to a dry Schlenk reaction tube -ketone (3.09g, 10.0mmol), pyridin-2-ylboronic acid dimethyl ester (1.66g, 11.0mmol), tetrakis(triphenylphosphine)palladium (0.12g, 0.1mmol), Na 2 CO 3 (2.12g, 20.0mmol), under the p...
Embodiment 2
[0088] Example 2: (Z)-5-(hydroxymethyl)-3-((5-(methylsulfonyl)-1H-benzo[d]imidazol-7-yl)methylene)-6-( Pyrimidin-2-yl)indolin-2-one (Compound II)
[0089]
[0090] According to the method of Example 1, with 1-(5-(methylsulfonyl)-1H-benzo[d]imidazol-7-yl)-formaldehyde instead of 1H-benzo[d]imidazole-7-formaldehyde, use 6-Bromo-5-(hydroxymethyl)indolin-2-one instead of 6-chloro-5-methylindolin-2-one, pyrimidin-2-ylboronic acid dimethyl ester instead of pyridin-2- Dimethyl boronic acid was used to obtain the title compound as a white solid in a total yield of 63.9% over two steps.
[0091] ESI-MS: 448.47[M+H] +
[0092] Elemental analysis: theoretical value / measured value, C(59.05 / 59.12), H(3.83 / 3.87), N(15.65 / 15.74), O(14.30 / 14.22), S(7.17 / 7.05)
[0093] 1 H NMR (400MHz, CDCl 3)δ12.37(s, 1H), 11.19(s, 1H), 8.81(d, 2H), 8.24(s, 1H), 8.12(s, 1H), 8.00(s, 1H), 7.76(s, 1H ), 7.56 (s, 1H), 7.39 (s, 1H), 7.28 (q, 1H), 5.23 (s, 1H), 4.63 (s, 2H), 3.31 (s, 3H).
[0094] Follo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com