Novel method for preparing trimethyliodosilane
A technology of trimethyl iodide silane and trimethyl chlorosilane, applied in the field of chemical synthesis, can solve the problems of complicated operation process, difficult operation, unsafety and the like, and achieve the effect of simple and safe process, easy operation and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
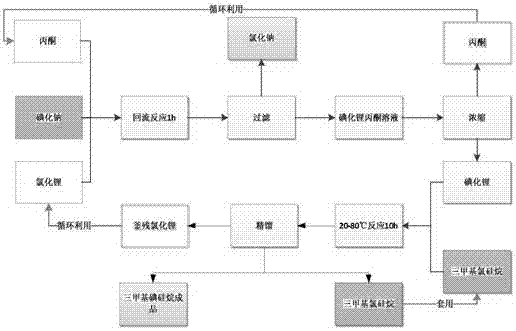
[0035] Experimental conditions: a) The molar ratio of anhydrous lithium chloride to anhydrous sodium iodide is 1:1, and the reaction temperature is 60°C; b) When iodosilane is synthesized, the ratio of lithium iodide to trimethylchlorosilane is 1: 2. The reaction temperature is 60°C, and the catalyst dosage is 2.5%.
[0036] (1) Preparation of anhydrous lithium iodide: add 107.32g sodium iodide (molar ratio 1:1) in portions to the 300ml acetone solution of 30.29g lithium chloride, react for 1 hour after dripping; Sodium chloride was removed; the filtrate was concentrated and evaporated to dryness to obtain lithium iodide, and after drying, 94 g (98.04%) of anhydrous lithium iodide was obtained, with a yield of 96%. The evaporated acetone is recovered and recycled after water removal.
[0037](2) Preparation of iodotrimethylsilane: place 94g lithium iodide, 154.78g trimethylchlorosilane (molar ratio 1:2) and 2.5% catalyst tetrabutylammonium iodide in a reaction flask under a n...
Embodiment 2
[0040] Experimental conditions: Example 1 reclaims lithium iodide and the recycling of trimethylchlorosilane, and other conditions are the same as Example 1. It is verified that recycling of materials can improve the yield of trimethyliodosilane, but has no effect on product quality.
[0041] (1) Preparation of iodotrimethylsilane: Lithium iodide 92g prepared by reclaiming lithium chloride in Example 1, 73.58g new trimethylchlorosilane, 77.57g example one steamed trimethylchlorosilane fraction and chlorine After washing with lithium chloride, the mother liquor of trimethylchlorosilane and 2.5% catalyst tetrabutylammonium iodide are placed in a reaction flask, under a nitrogen atmosphere, and reacted at a certain temperature for 10 hours to obtain a crude product of iodotrimethylsilane, which is obtained after rectification The finished product of iodotrimethylsilane is 133.22g, the purity is 99.32%, and the yield is 98.56%.
[0042] (2) recycling of lithium chloride: prepare 3...
Embodiment 3
[0044] Experimental conditions: a) The molar ratio of anhydrous lithium chloride to anhydrous sodium iodide is 1.05:1, and the reaction temperature is 60°C; b) When iodosilane is synthesized, the ratio of lithium iodide to trimethylchlorosilane is 1: 1.2, the reaction temperature is 60°C, and the catalyst consumption is 2.5%.
[0045] (1) Preparation of anhydrous lithium iodide: Add 151.19 g of sodium iodide (1.05:1) in portions to 44.81 g of lithium chloride in acetone, and react for 1 hour after the drop; after the reaction is completed, filter out the chloride Sodium; the filtrate was concentrated and evaporated to dryness to obtain lithium iodide, and after drying, 134.12 g (97.80%) of anhydrous lithium iodide was obtained, with a yield of 98%. The evaporated acetone is recovered and recycled after water removal. The feed ratio of anhydrous lithium chloride to anhydrous sodium iodide is 1.05:1, and the conversion rate of lithium iodide is improved compared with Example 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com