Limonin derivatives and preparation methods and medical applications thereof
A use and pharmaceutical technology, applied to water-soluble limonin derivatives for the treatment of pain and inflammation, can solve the problems of insufficient effect, low bioavailability, poor water solubility, etc., to eliminate inflammation, relieve pain, and relieve pain. pain effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
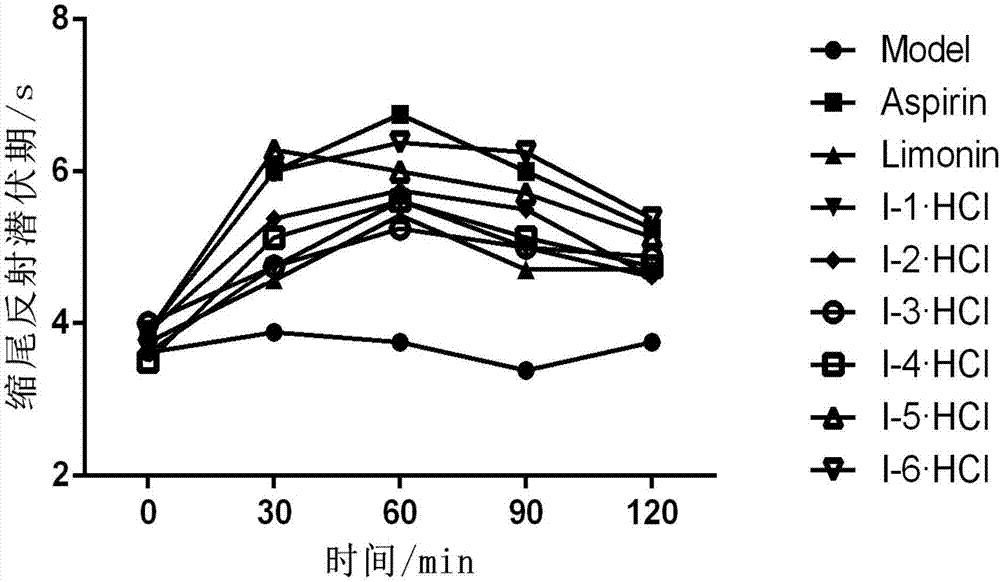
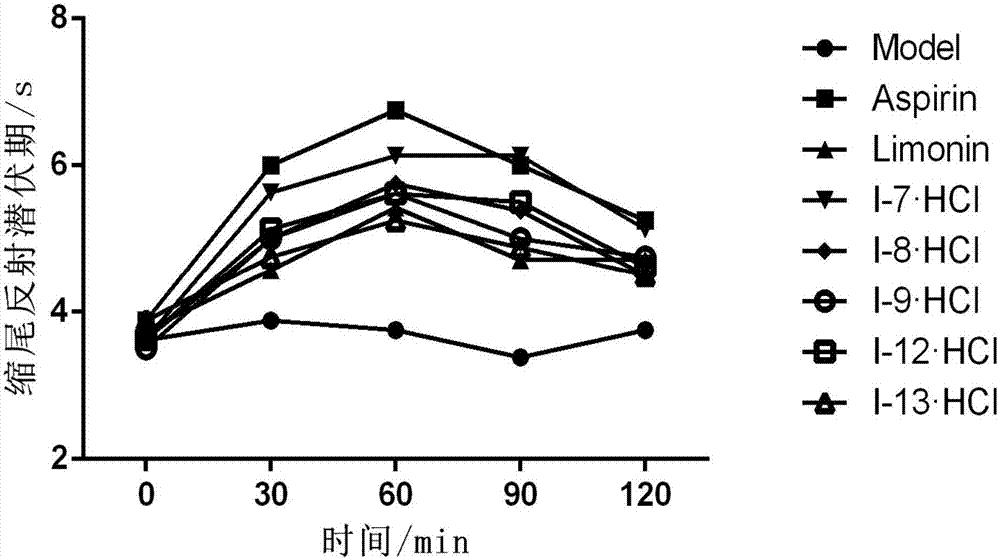
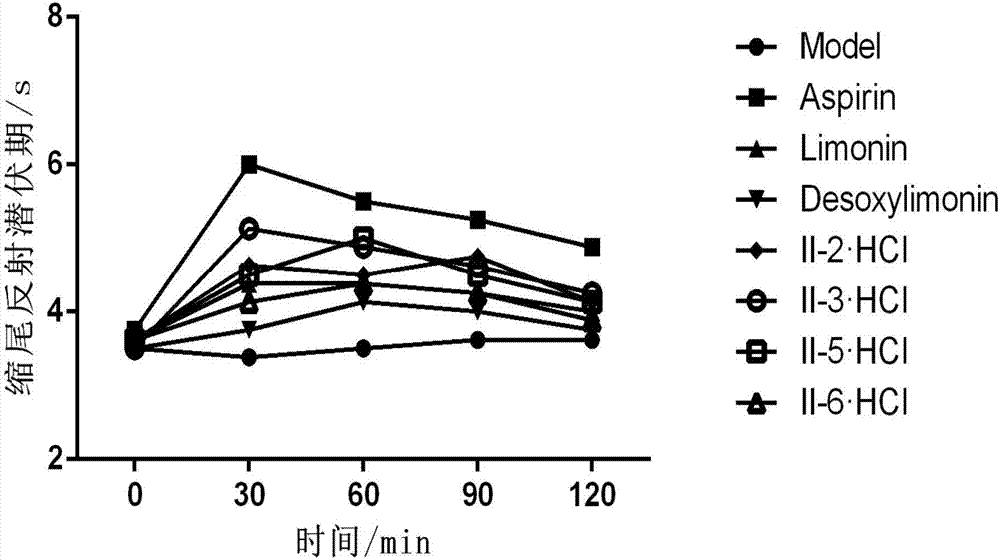
Image
Examples
Embodiment 1
[0108] Preparation of 23-(2-(piperidin-1-yl)acetyl)limonoid (I-1)
[0109] 23-Chloroacetyllimonoid (IV-1)
[0110] Add limonin (8g, 17mmol) and dichloromethane (125mL) into a 250mL three-necked bottle, stir and cool down to 0°C, add aluminum trichloride (10.2g, 76.5mmol) in batches, after the addition is complete, keep stirring for 20 Minutes, dropwise added a mixed solution of chloroacetyl chloride (2.2g, 19.5mmol) and dichloromethane (5mL), after dropping, kept at 0~-5°C for 1 hour, then continued stirring at room temperature for 3 hours, then stopped the reaction. Slowly pour the reaction solution into 100 mL of ice water, separate the dichloromethane layer, extract the water layer twice with dichloromethane (100 mL×2), combine the organic layers, and wash three times with 1 mol / L sodium hydroxide aqueous solution (100 mL×2). 3), washed twice with saturated brine (100 mL×2), dried over anhydrous sodium sulfate, filtered with suction, and evaporated to remove the solvent un...
Embodiment 2
[0115] Preparation of 23-(2-(morpholin-4-yl)acetyl)limonoid (I-2)
[0116] Using compound IV-1 (2.0g, 3.65mmol) and morpholine (0.95g, 10.90mmol) as raw materials, the operation process was the same as I-1, and the crude product was purified by column chromatography (dichloromethane:methanol=90:1), 1.2 g of light yellow solid (I-2) was obtained, yield 54.9%, 146° C. (charring). 1 H NMR (300MHz, CDCl 3 )δ7.59(s,1H),7.26(s,1H),5.51(s,1H),4.79(d,J=13.1Hz,1H),4.49(d,J=13.2Hz,1H),4.06( s,2H),3.85–3.73(m,4H),3.70(s,2H),3.00(dd,J=16.8,3.7Hz,1H),2.95–2.79(m,1H),2.78–2.57(m, 5H),2.57–2.42(m,2H),2.24(dd,J=15.8,3.1Hz,1H),1.91–1.78(m,3H),1.62–1.46(m,1H),1.32(s,3H) ,1.20(s,3H),1.17(s,3H),1.10(s,3H).ESI-MS(m / z):598.3[M+H] +
[0117] Using compound I-2 (1.0g, 1.67mmol) as raw material, the operation process was the same as I-1·HCl to obtain 0.87g of light yellow solid (I-2·HCl), yield 83%, m.p.>200℃.
Embodiment 3
[0119] Preparation of 23-(2-(piperazin-1-yl)acetyl)limonoid (I-3)
[0120] Using compound IV-1 (2.0g, 3.65mmol) and N-tert-butoxycarbonylpiperazine (2.73g, 14.6mmol) as raw materials, the operation process was the same as I-1, and the crude product was subjected to column chromatography (dichloromethane:methanol = 150:1) purification to obtain 1.46g light yellow intermediate. Dissolve the intermediate in methanol (20mL), add saturated HCl in methanol (20mL) dropwise with stirring in an ice bath, after the dropwise reaction, react at room temperature for 2 hours, and detect by TLC (developing solvent: dichloromethane:methanol=25:1) After the reaction was complete, it was placed at 0-5°C for 1 hour, and 0.9 g of a light yellow solid (I-3) was obtained by suction filtration. The total yield of the two steps was 41.2%. 1 H NMR (300MHz, CDCl 3 )δ7.57(s,1H),7.25(s,1H),5.49(s,1H),4.77(d,J=12.7Hz,1H),4.47(d,J=13.4Hz,1H),4.03( s,2H),3.68(s,2H),3.06(s,4H),2.97(dd,J=16.8,3.9Hz,1H),2.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com