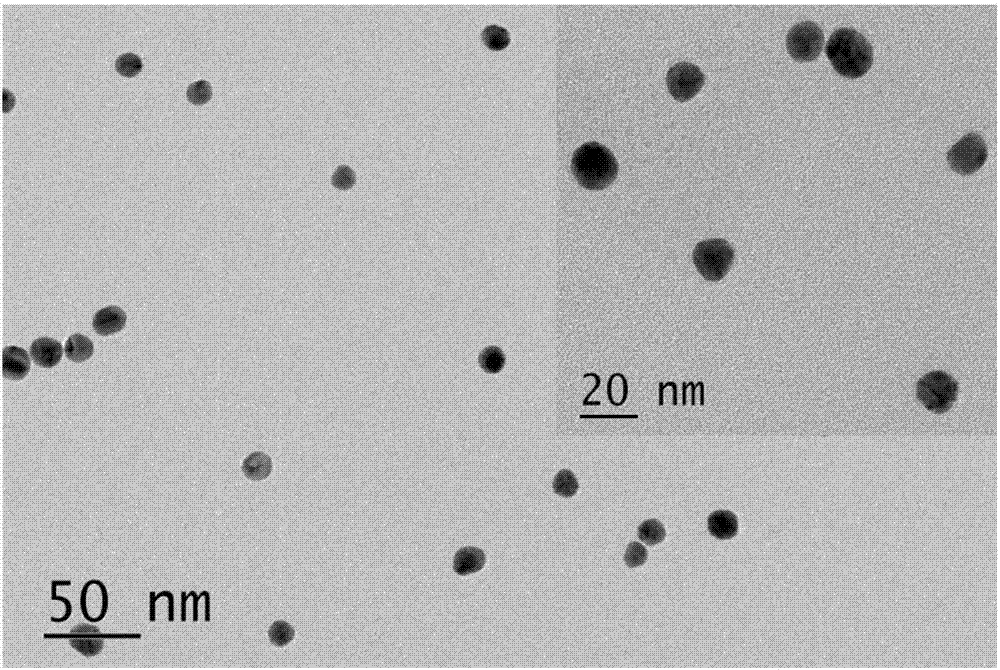
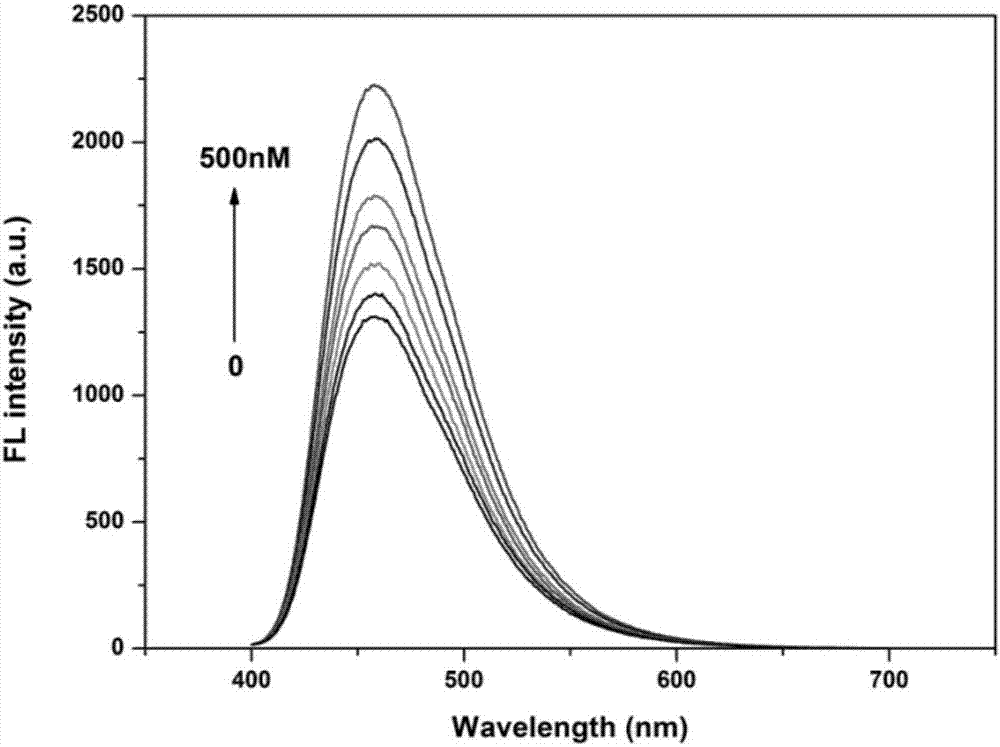
Method of detecting adenosine with fluorescent sensor on the basis of aptamer
An aptamer sensor and fluorescence sensor technology, applied in the field of analysis and detection, can solve the problems affecting the sensitivity and selectivity of the sensor, and achieve the effects of facilitating in vitro screening and preparation, reducing background fluorescence and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] a) Construction of fluorescent aptamer sensor: Mix 45 μLaptamer-AuNPs and 30 μL ssDNA-CDs evenly, add 105 μL 10 mmol / L pH7.4 phosphate buffer, and incubate at 37°C for 30 min;
[0035] b) Detection of adenosine by fluorescent sensing: Add 20 μL of OnM, 0.1 μM, 0.25 μM, 1.5 μM, 2.5 μM, 3.5 μM, 5 μM adenosine solutions to the sensor constructed in step a), incubate at room temperature for 30 min, and measure the respective Fluorescence intensity value F, while measuring the blank fluorescence value F0 , as the relative fluorescence intensity [(F-F 0 ) / F 0 ] standard curve corresponding to the adenosine concentration;
[0036] c) Fluorescent detection of adenosine: Add a solution of unknown adenosine concentration to the sensor constructed in step a), measure its fluorescence intensity value, and obtain the concentration of adenosine in the solution to be tested according to the standard curve in step b). Measure the fluorescence intensity of the system at 460nm, the exc...
Embodiment 2
[0039] a) Construction of fluorescent aptamer sensor: Mix 50 μLaptamer-AuNPs and 25 μL ssDNA-CDs evenly, add 110 μL 20 mmol / L pH 7.0 phosphate buffer, and incubate at 37°C for 60 min;
[0040] b) Detection of adenosine by fluorescent sensing: Add 15 μL of OnM, 0.1 μM, 0.25 μM, 1.5 μM, 2.5 μM, 3.5 μM, 5 μM adenosine solutions to the sensor constructed in step a), incubate at room temperature for 45 min, and measure the fluorescence intensity values to draw a standard curve.
[0041] c) Fluorescent detection of adenosine: Add a solution of unknown adenosine concentration to the sensor constructed in step a), measure its fluorescence intensity value, and obtain the concentration of adenosine in the solution to be tested according to the standard curve in step b). Measure the fluorescence intensity of the system at 460nm, the excitation wavelength is 365nm, and the scanning speed is 1200nm min -1 , the voltage of the photomultiplier tube is 700V, and the width of the excitation...
Embodiment 3
[0044] a) Construction of fluorescent aptamer sensor: Mix 50 μLaptamer-AuNPs and 50 μL ssDNA-CDs evenly, add 80 μL 25 mmol / L pH7.5 phosphate buffer, and incubate at 37°C for 45 min;
[0045] b) Detection of adenosine by fluorescent sensing: Add 20 μL of OnM, 0.1 μM, 0.25 μM, 1.5 μM, 2.5 μM, 3.5 μM, 5 μM adenosine solutions to the sensor constructed in step a), incubate at room temperature for 30 min, and measure the fluorescence intensity values to draw a standard curve.
[0046] c) Fluorescent detection of adenosine: Add a solution of unknown adenosine concentration to the sensor constructed in step a), measure its fluorescence intensity value, and obtain the concentration of adenosine in the solution to be tested according to the standard curve in step b).
[0047] The above adenosine nucleic acid aptamer sequence is 5'-SH-C 6 -AGA GAA CCT GGG GGAGTA TTG CGG AGGAAG GT-3', the adapter complementary strand sequence is 5'-NH 2 -C 6 -ACC TTC CTC CGC-3'.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com