Polypeptide of scorpion toxin and synthetic method thereof
A synthesis method and technology of scorpion toxin, applied in the field of scorpion toxin polypeptide and its synthesis, can solve the problems of easy occurrence of mismatch and low renaturation efficiency, and achieve the effects of stable protein spatial conformation, simple operation and high activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of diaminodiacid building blocks
[0029] The reaction formula is:
[0030]
[0031] The specific steps are:
[0032] Compound 2: Take 1.5g (12.6mmol) of homoserine and 1.34g (12.6mmol) of sodium carbonate in a 150mL round-bottom flask, and dissolve them with 30mL of water and 15mL of 1,4-dioxane. The reaction system was stirred at 0°C, and 4.26 g (12.6 mmol) fluorenylmethoxycarbonyl succinimide (Fmoc-OSu) was added in batches. After the addition is complete, return the reaction system to room temperature (if there is undissolved solid in the reaction system, add an appropriate amount of 1,4-dioxane to dissolve it), and stir overnight. The 1,4-dioxane in the solvent was removed with a rotary evaporator, after which the remaining aqueous phase was extracted with ethyl acetate. Keep the aqueous phase and adjust the pH to 2 with 6M hydrochloric acid solution. The pH-adjusted aqueous phase was extracted with ethyl acetate, the organic phases were combined...
Embodiment 2
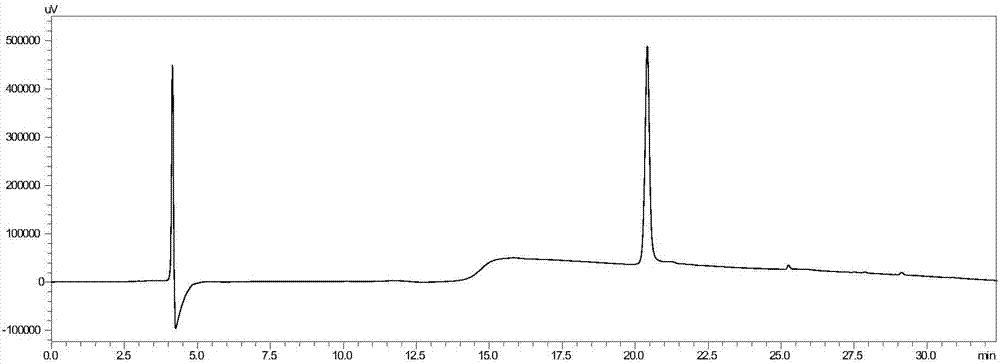
[0043] Preparation of Scorpion Toxin Polypeptide
[0044] The amino acid sequence of the scorpion toxin polypeptide is shown in SEQ ID No.1, and the S-S bond between the 8th and 28th positions is replaced by a C-S bond, which is equivalent to the S atom on Cys at the 28th position replaced by C atoms.
[0045] The reaction formula is:
[0046]
[0047] The specific steps are:
[0048] Weigh Rink AM amide Resin (0.1mmol) in a solid-phase synthesis tube, use DMF / DCM mixed solvent (1:1 volume ratio) to swell for 15 minutes, drain it, and then synthesize the above-mentioned Scorpion toxin polypeptide (0.4mmol amino acid, 0.4mmol Oxyma, 0.4mmol DIC; reaction time is 20-30min, reaction temperature is 75°C), in which arginine (Arg) uses HCTU as a condensation agent, and reacts twice at room temperature for 40min; Amino acid (His) uses DIC as the condensing agent, reacts at room temperature for 40 minutes; cysteine (Cys) uses DIC as the condensing agent, reacts at 50°C for 40 ...
Embodiment 3
[0054] Activity Test of Scorpion Toxin Polypeptide
[0055] CHO cell culture and transfection process
[0056] Chinese hamster ovary (CHO) cells have no background current of potassium ion channels, and will not cause interference when studying potassium ion channels.
[0057] Cell culture medium: DMEM / F12 medium (Gibco Company) used for culturing cells. Add 10% fetal bovine serum (FBS) (Gibco) and 100 U / mL penicillin and streptomycin (Gibco) before use.
[0058] Cell Recovery: Turn on the 37 °C water bath. Prepare DMEM / F12 medium containing serum FBS and double-resistant penicillin and streptomycin, and place it in a 37°C water bath, preheating to 37°C. Take the cells out of the liquid nitrogen irrigation, put them into a water bath at 37 degrees Celsius quickly, put them into a centrifuge after they melt, centrifuge at 1000rpm for 5min, and discard the supernatant. The cells were resuspended with 1 mL of preheated DMEM / F12 medium, added to a 60 mm culture dish, and 2 mL ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com